Question
Question: Among the following, the correct statement(s) is (are): (A) \[{\rm{B}}{{\rm{H}}_{\rm{3}}}\]has thr...
Among the following, the correct statement(s) is (are):
(A) BH3has three -centre two electron bonds in its dimeric structure
(B) Al(CH3)3has three-centre two electron bonds in its dimeric structure
(C) AlCl3has three-centre two electron bonds in its dimeric structure
(D) The Lewis acidity of BCl3is greater than that of AlCl3
Solution
As we know that, Lewis acids are those acids which are deficient in electrons, so these compounds are always found in dimer. In dimeric structures, electrons are always shared between the atoms. As we go down the group, the size of the orbital increases.
Complete step by step answer
As we know that boron and aluminium are found in 13th group and aluminium is the second element of this group. Boron and aluminium have three electrons in its outermost shell.
Let’s move towards options:
->BH3 has six electrons in its octet and to complete its octet, it forms dimer. Boron atom shares one electron of the outermost shell to hydrogen to form bridged hydrogen bonding. Another boron atom also shares its electron to form bridging bonds with hydrogen as shown below.
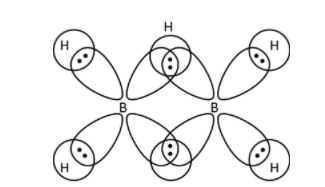
So, we can see that each bridged bond contains two electrons bonded with three atoms. Therefore, option (A)- BH3 has three -centre two electron bonds in its dimeric structure is correct.
->Al(CH3)3 has six electrons in its octet and to complete its octet, it forms dimer. Aluminium atoms share one electron of the outermost shell with one electron of carbon to form bridged bonding. Other aluminium atoms also share their electrons to form bridging bonds with carbon atoms as shown below.
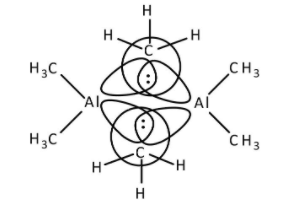
Therefore, option (B)- Al(CH3)3 has three centre- two electron bonds in its dimeric structure is correct.
->Option (C)- AlCl3 also forms dimeric structure because it also has six electrons in its octet and to complete its octet, it forms dimer. One aluminium atom shares one electron of outermost shell with one electron of chlorine atom to form bridged bonding and chlorine also donate its one lone pair of electrons to vacant orbital of aluminium atom to form coordinate bonding as shown below.
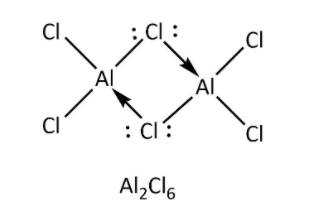
As we can see, bridging bonding contains three atoms with four electrons, so it does not form three centre -two electron bonds.
Therefore, this option is incorrect.
->Now, option (D)- The Lewis acidity of BCl3 is greater than that of AlCl3 is correct because boron’s 2p-orbital overlaps with chlorine’s 3p-orbital, hence, cannot form bond effectively. Therefore, Lewis acidity is greater. On the other hand, in AlCl3, overlapping is 3p−3p between aluminium and chlorine.
**Therefore, the correct options are option (A), option (B) and option (D).
Note: **
From IR spectra, stretching frequency of bridging bonds is less than terminal bonds because electron density is less than the volume. Compounds like Al2Cl6 are very unique because this kind of structure is very rare.
