Question
Question: Among the following, the compound having the highest enol content is: A.acetone B.acetophenone...
Among the following, the compound having the highest enol content is:
A.acetone
B.acetophenone
C.acetyl acetone
D.acetic acid
Solution
The enol content refers to the stability of the tautomer product of a carbonyl group.
-Stability depends on the number of resonance structures. More the number of resonance structures, higher is the stability and hence, more will be its enol content.
Complete step by step answer:
Let us first understand the terms in the question. Enol refers to a double bond which is in conjugation with an alcohol group. 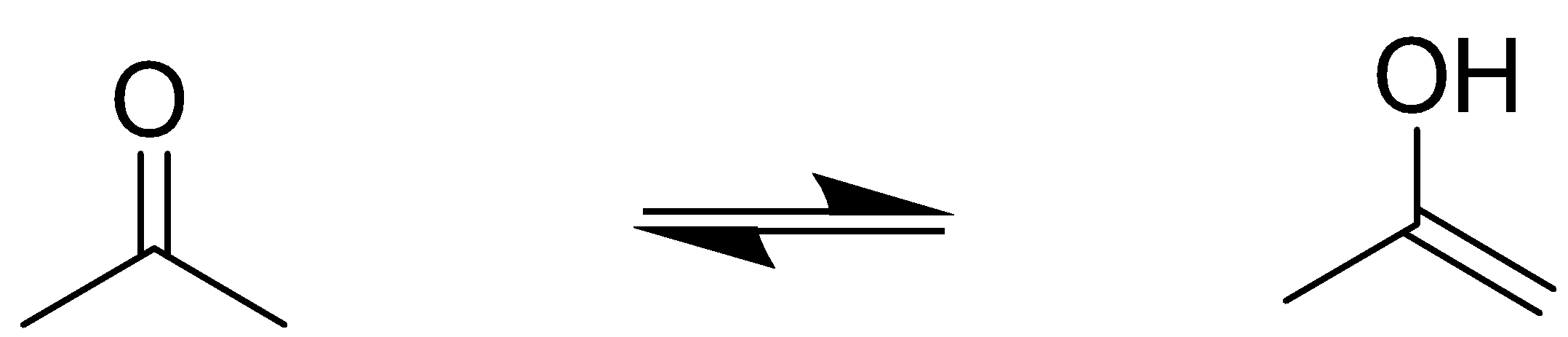 From this above example, we can see that a hydrogen atom migrates to the carbonyl oxygen to form alcohol and the hydrogen deficient carbon forms a double bond. This process is called tautomerization and the enol product is a tautomer of the carbonyl reactant. The structures of the given compounds are:
From this above example, we can see that a hydrogen atom migrates to the carbonyl oxygen to form alcohol and the hydrogen deficient carbon forms a double bond. This process is called tautomerization and the enol product is a tautomer of the carbonyl reactant. The structures of the given compounds are: 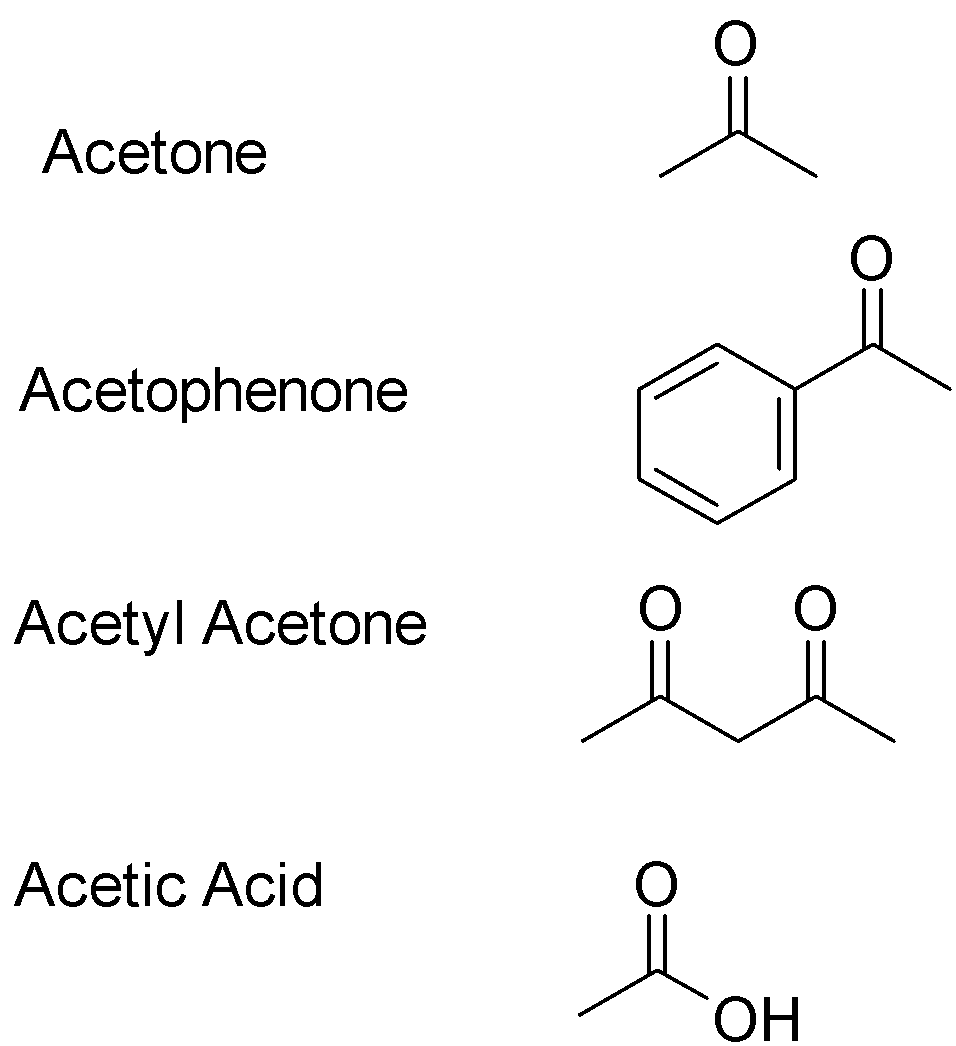
Enol content means how much of the compound would be present in its enol form in a pure solution of the compound. If the reaction favors the enol product, the enol content would be great i.e. enol has to be more stable than the carbonyl group. Ketonic form predominates in simple mono carbonyl compounds like acetone, acetophenone and ethanoic acid . This is due to the greater strength of C=O present in keto form than C=C bond present in enol form. Enolic form presents in 1,3-dicarbonyl compounds like acetyl acetone Out of the given options, acetyl acetone will have the highest enol content due to the stability of the enol product. Hence correct answer is optionC.
Note:
Tautomerization is similar to resonance. It only involves the migration of a proton like there is a charge migration in resonance. Tautomerization does not lead to the formation of a new product. Every carbonyl compound will have some percentage of the compound in its enol form in the solution.
