Question
Question: Among the following species : \({\text{N}}{{\text{F}}_{\text{3}}}{\text{,N}}{{\text{O}}_{\text{3}}}^...
Among the following species : NF3,NO3 - ,BF3,H3O + ,HN3 , the isostructural pairs are:
(A) [NF3,NO3 - ] and [BF3,H3O + ]
(B) [NF3,HN3] and [NO3 - ,BF3]
(C) [NF3,H3O + ] and [NO3 - ,BF3]
(D) [NF3,H3O + ] and [HN3,BF3]
Solution
Isostructural species are those species which have the same shape and shape hybridization. The geometry of molecules is fixed for a particular hybridization but the shapes may vary depending upon the presence or absence of lone pairs. Therefore, the isostructural species can be identified when their exact shapes are known.
Complete step by step answer:
We are given 5 species NF3,NO3 - ,BF3,H3O + ,HN3 and we need to find out the isostructural pairs from these. Let us at first check the structures of these 5 species. NF3 is a trihalide of nitrogen of group 15. Like ammonia, the trihalides of group 15 elemenst also have pyramidal structures. So, NF3 will also have a pyramidal structure, i.e., the central element nitrogen is sp3 hybridized. Three of the four sp3 orbitals form sigma bonds with fluorine atoms and the fourth sp3 orbital contain the lone pair of electrons. The lone pair-bond pair repulsion is greater than the bond pair-bond pair repulsion due to which the expected tetrahedral shape gets distorted and the bond angle becomes less than the tetrahedral bond angle 109∘ and the structure becomes pyramidal.
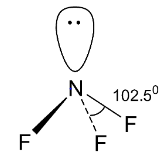
NO3 - has the following structure.
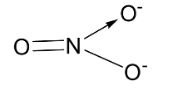
Here, the central element of the molecule which is nitrogen is sp2 hybridized. The 3 sp2 hybridized orbitals of nitrogen overlaps with one s orbital of oxygen and the nitrogen p orbital makes a double bond with 3 oxygen atoms. Thus, nitrogen is bonded to 3 oxygen atoms and no lone pairs are present and the oxygen atoms lie at the corners of a triangle at the same plane. So the molecule will have slightly bent geometry with bond angle 120∘ and trigonal planar structure.
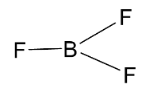
Next, we have BF3 molecule which has the following structure.
Here, the central element of the molecule which is boron is sp2 hybridized and so the molecule will have a trigonal planar structure. Here, the three sp2 hybridized orbitals of boron atoms overlap with p-orbitals of 3 fluorine atoms. All the boron orbitals are arranged in triangular shape and all the atoms lie on the same plane. So, it has a trigonal planar structure with bond angle 120∘ .
Next, the hydronium ion has the following structure.
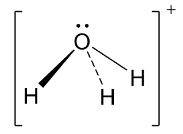
Thus, the central element of the hydronium ion which is oxygen is sp3 hybridized. So, ideally it should have tetrahedral structure. But, in this case, there are 3 bond pairs and one lone pair of electrons which causes distortion leading to pyramidal shape and bond angle becomes 109.5∘.
Lastly, we have hydrazoic acid HN3 which is a special hydride of nitrogen. It has the structure as shown below.
Here, nitrogen is sp hybridized and hence hydrazoic acid will have linear structure with bond angle 180∘ .
From the above discussion, we can see that [NF3,H3O + ] have similar hybridization and structures and [NO3 - ,BF3] have similar hybridization and structures.
So, the correct option is C.
Note: Isoelectronic species are those species which have the same number of electrons around them. For example, the fluoride ion, neon and sodium ion have 10 electrons around them and so they are isoelectronic. Species which are isoelectronic usually have similar structures and so they are also isostructural. For example, methane and ammonium ion are isoelectronic as well as isostructural as both have tetrahedral structure.
