Question
Question: Among the following species identify the isostructural pairs: \(N{{F}_{3}},NO_{3}^{-},B{{F}_{3}},{...
Among the following species identify the isostructural pairs:
NF3,NO3−,BF3,H3O+,HN3
(i)[NF3,NO3−] and [BF3,H3O+]
(ii)[NF3,HN3] and [NO3−,BF3]
(iii)[NF3,H3O+] and [NO3−,BF3]
(iv)[NF3,H3O+] and [HN3,BF3]
Solution
Two compounds can only be isostructural when they have same degree of hybridization along with same number of lone pairs there central atom contains, As structure of a molecule will always be decided on the fact that how many atomic orbitals need to be accommodate and how much repulsion they have to face.
Complete step by step answer:
Degree of hybridization = number of sigma bonds + number of lone pairs
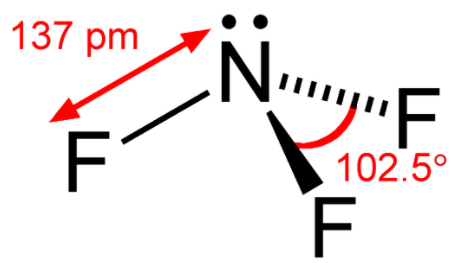
Structure of NF3: It has nitrogen as a central atom with three fluorine atoms forming three single bonds with it.
Electronic configuration of Nitrogen: 1s22s22p3
Clearly nitrogen has three half filled orbitals that will form 3 single bonds with three fluorine atoms and it also has one lone pair.
Degree of hybridization= 3 + 1
Degree of hybridization = 4
So NF3 molecule will have sp3 hybridization with one lone pair present in the molecule, that’s why its structure will be pyramidal.
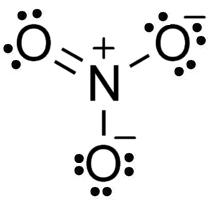
Structure of NO3−: It has nitrogen as a central atom with three oxygen atoms forming bonds with it.
Electronic configuration of Nitrogen: 1s22s22p3
Clearly nitrogen has three half filled orbitals that will form bonds with 3 oxygen atoms and to accommodate them nitrogen atom has to show its maximum covalency 4 and with one oxygen it has to form a pi bond and its lone pair will also take part in bond formation.
Degree of hybridization= 4 + 0
Degree of hybridization = 4
So NO3− ion will have sp2 with three sigma bonds and one pi bond present in the ion, that’s why its structure will be trigonal planar with bond angle1200
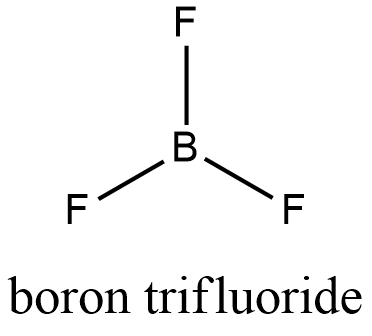
- Structure of BF3: It has boron as a central atom with three fluorine atoms forming three single bonds with it.
Electronic configuration of boron: 1s22s22p1
To make 3 sigma bonds boron needs to have 3 half filled orbitals, one of the 2S electron will move into vacant 2p orbital
New electronic configuration of boron: 1s22s12p2
Clearly Boron has three half filled orbitals that will form 3 single bonds with three fluorine atoms.
Degree of hybridization= 3 + 0
Degree of hybridization = 3
So BF3 molecule will have sp2 hybridization with three bonds present in the molecule, that’s why its structure will be trigonal planar structure with bond angle 1200
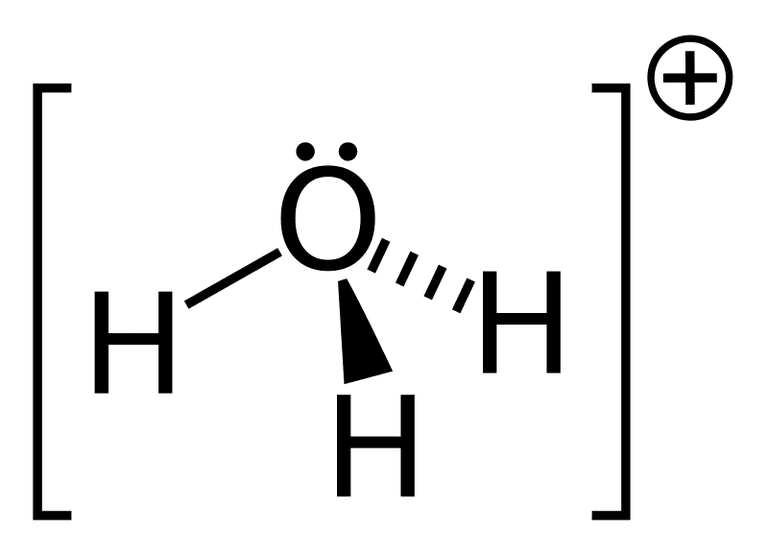
- Structure of H3O+: It has oxygen as a central atom with three hydrogen atoms forming three single bonds with it.
Electronic configuration of Oxygen: 1s22s22p4
Clearly Oxygen has two half filled orbitals that will form 2 single bonds but it has to accommodate third hydrogen as well so one of the lone pairs is going to take part in bond formation and therefore oxygen will have only one lone pair with it.
Degree of hybridization= 3 + 1
Degree of hybridization = 4
So H3O+ ion will have sp3 hybridization with one lone pair present in the ion, that’s why its structure will be pyramidal with bond angle 1130
Structure of HN3: It has nitrogen atoms.
Electronic configuration of Nitrogen: 1s22s22p3
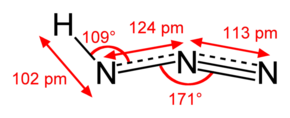
As you can see in the structure, central nitrogen atom is sp hybridized as bond angle is close to 1800 while terminal nitrogen atoms are sp2 hybridized where bond angle is close to 1200
- So here N3− will have a linear structure where hydrogen ion is bonded to one of the terminal nitrogen atoms in the same plane.
- From the above discussion we can clearly see that NO3− and BF3 are isostructural and NF3 and H3O+ ion are isostructural.
The correct answer is option “C” .
Additional Information : N3H is called hydrazoic acid. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride.
Note: Repulsion between lone pair and lone is highest and after that lone pair and bond pair repulsion value comes and repulsion between bond pair and bond pair is minimum. That is the reason why the presence of lone pairs always distorts the structure and bond angle decreases than the expected value to minimize the repulsion.
