Question
Question: Among the following reactions, identify the INCORRECT ones....
Among the following reactions, identify the INCORRECT ones.
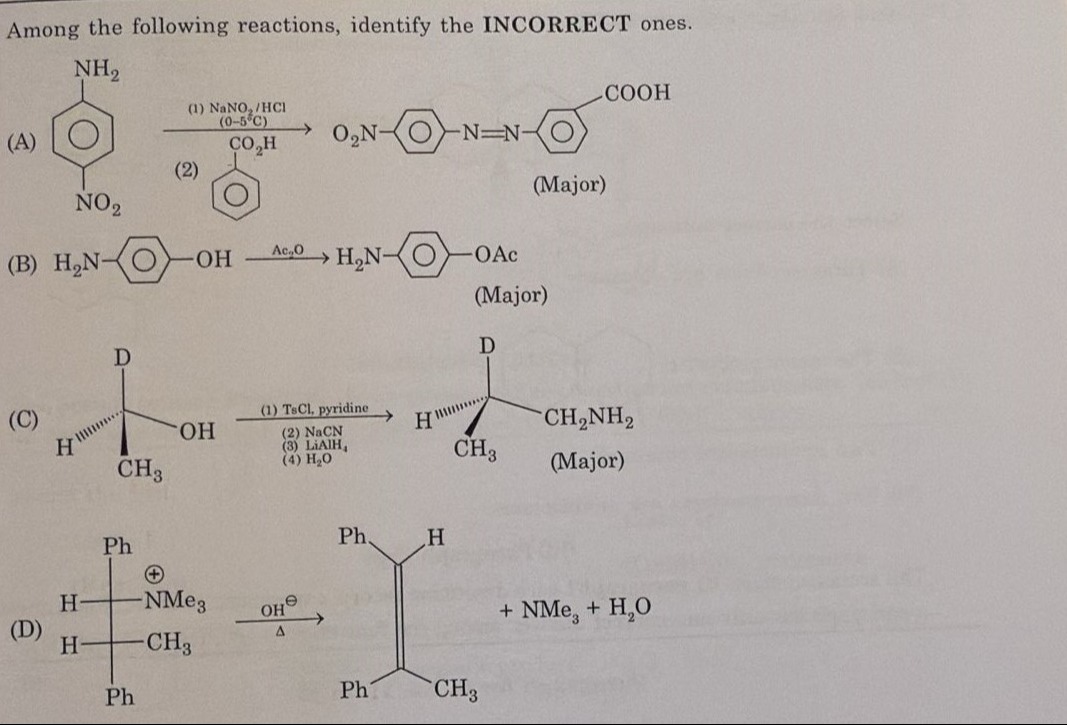
\begin{array}{c} \text{NH}_2\\ \includegraphics[width=0.1\textwidth]{circle.png}\\ \text{NO}_2 \end{array} \xrightarrow{\begin{subarray}{c} (1) \text{ NaNO}_2/\text{HCl} \\ (0-5^\circ\text{C})\\ \text{CO}_2\text{H}\\ (2) \end{subarray}} \text{O}_2\text{N} \includegraphics[width=0.1\textwidth]{circle.png} \text{N=N} \includegraphics[width=0.1\textwidth]{circle.png} \text{COOH} \text{(Major)}
\text{H}_2\text{N} \includegraphics[width=0.1\textwidth]{circle.png} \text{OH} Ac2O \text{H}_2\text{N} \includegraphics[width=0.1\textwidth]{circle.png} \text{OAc} \text{(Major)}
\begin{array}{c} \text{D}\\ \includegraphics[width=0.1\textwidth]{figure.png}\\ \text{CH}_3 \end{array} \xrightarrow{\begin{subarray}{c} (1) \text{ TsCl, pyridine}\\ (2) \text{ NaCN}\\ (3) \text{ LiAlH}_4\\ (4) \text{ H}_2\text{O} \end{subarray}} \begin{array}{c} \text{D}\\ \includegraphics[width=0.1\textwidth]{figure2.png}\\ \text{CH}_3 \end{array} \text{CH}_2\text{NH}_2 \text{(Major)}
\begin{array}{c} \text{Ph}\\ \includegraphics[width=0.1\textwidth]{figure3.png}\\ \text{Ph} \end{array} \xrightarrow{\begin{subarray}{c} \text{OH}^\ominus\\ \Delta \end{subarray}} \begin{array}{c} \text{Ph}\\ \includegraphics[width=0.1\textwidth]{figure4.png}\\ \text{Ph} \end{array} \text{+ NMe}_3\text{ + H}_2\text{O}
A, B, C, D
Solution
Let's analyze each reaction.
(A) This reaction is a diazotization of 4-nitroaniline followed by azo coupling with benzoic acid.
-
Step 1: Diazotization of 4-nitroaniline gives 4-nitrophenyldiazonium chloride.
O2N−C6H4−NH2NaNO2/HCl,0−5∘CO2N−C6H4−N2+Cl−
-
Step 2: Azo coupling is an electrophilic aromatic substitution reaction where the diazonium ion is the electrophile. Coupling occurs with activated aromatic rings. Benzoic acid is deactivated towards electrophilic substitution due to the electron-withdrawing COOH group, which is meta-directing. The product shown is formed by coupling at the para position of benzoic acid. Azo coupling with deactivated rings like benzoic acid is generally not favorable under standard conditions and the product shown, coupled at the para position to COOH, is unlikely to be the major product. Azo coupling typically occurs with phenols and amines (activated rings). Therefore, reaction (A) is incorrect.
(B) This is the reaction of 4-aminophenol with acetic anhydride (Ac2O). Acetic anhydride acetylates nucleophilic groups like amines and alcohols. The amino group is generally more nucleophilic than the hydroxyl group. Therefore, the major product is expected to be formed by acetylation of the amino group, yielding 4-acetylaminophenol (H3C−CO−NH−C6H4−OH). The product shown is 4-acetoxyaniline (H2N−C6H4−OAc), where the hydroxyl group is acetylated. While acetylation of the hydroxyl group is possible, it is usually the minor product compared to acetylation of the amino group under these conditions. Therefore, reaction (B) is incorrect as the major product.
(C) This reaction involves a chiral alcohol. Let's determine the configuration of the starting alcohol. The chiral carbon is bonded to OH, D, H, and CH3. Priorities are OH (1), D (2), CH3 (3), H (4). With OH wedge, CH3 dash, and H, D in the plane, the configuration is (S).
- Step 1: Reaction with TsCl/pyridine converts the alcohol to a tosylate with retention of configuration, giving (S)-tosylate.
- Step 2: Reaction with NaCN is an SN2 reaction, which causes inversion of configuration. (S)-tosylate reacts with CN− to form (R)-nitrile.
- Step 3: Reduction of the nitrile with LiAlH4 converts CN to CH2NH2. This step does not involve the chiral center, so the configuration remains (R).
- Step 4: Hydrolysis is the workup step.
The final product is an amine with the chiral center having configuration (R). The product shown has the chiral carbon bonded to CH2NH2, D, H, CH3. Priorities are CH2NH2 (1), D (2), CH3 (3), H (4). With CH2NH2 wedge, CH3 dash, and H, D in the plane, the configuration is (S). Since the expected product is (R), reaction (C) is incorrect.
(D) This is a Hofmann elimination reaction of a quaternary ammonium hydroxide salt upon heating. The leaving group is trimethylamine (NMe3). The reaction follows the Hofmann rule, where elimination occurs to form the least substituted alkene. The quaternary ammonium salt is derived from 2-phenyl-3-methylbutan-2-amine. The structure shows the quaternary ammonium salt as (2R,3R)-N,N,N-trimethyl-2,3-diphenylbutan-2-aminium. The hydrogen atoms available for elimination are on the carbon adjacent to the carbon bearing the positive nitrogen. These are the hydrogens on the methyl group (CH3) and the hydrogen on the tertiary carbon bearing the methyl and phenyl groups. Elimination of a proton from the methyl group would lead to a less substituted alkene (vinyl group attached to the chiral center), while elimination from the tertiary carbon would lead to a more substituted alkene (double bond between the two phenyl-substituted carbons). According to the Hofmann rule, the least substituted alkene is formed. However, the product shown is 1,2-diphenylbut-1-ene, which is a more substituted alkene. Let's re-examine the structure of the reactant and product. The reactant is (2R,3R)-N,N,N-trimethyl-2,3-diphenylbutan-2-aminium. The carbons adjacent to the nitrogen are C1 (methyl group), C2 (the chiral carbon bonded to N+), and C3 (the other chiral carbon). C1 has hydrogens. C3 has a hydrogen. Elimination can occur between C2 and C1 or between C2 and C3. Elimination between C2 and C1 would produce an alkene with a vinyl group. Elimination between C2 and C3 would produce an alkene with the double bond between C2 and C3. The product shown is 1,2-diphenylbut-1-ene. Let's number the carbons in the reactant: Ph−C(+NMe3)−C(Ph)(CH3)−H. This numbering doesn't match the name. Let's assume the name is correct and the structure represents it. 2,3-diphenylbutan-2-amine. The quaternary salt is derived from this amine. The nitrogen is at position 2. So we have Ph−CH(Ph)−C(CH3)(NMe3+)−H. This would be 2,3-diphenylbutan-2-aminium. The structure in the question shows Ph−CH(H)−C(Ph)(NMe3+)−CH3. This is 2,3-diphenylbutan-2-aminium. The carbons adjacent to the nitrogen-bearing carbon (C2) are C1 (methyl) and C3 (tertiary carbon with H and Ph). Elimination can occur from C1 or C3. Elimination from C1 gives Ph−CH(Ph)−C(=CH2)−H. This is 2,3-diphenylbut-1-ene. Elimination from C3 gives Ph−C(=C(Ph)(CH3))−H. This is 1,2-diphenylbut-1-ene. According to the Hofmann rule, the less substituted alkene is favored, which is 2,3-diphenylbut-1-ene. The product shown is 1,2-diphenylbut-1-ene. This is the more substituted alkene, which is the Saytzeff product, not the Hofmann product. Therefore, reaction (D) is incorrect as the major product of Hofmann elimination.
All four reactions (A), (B), (C), and (D) are incorrect as described or as the major product. The question asks to identify the incorrect ones. Since all of them are incorrect, all options (A), (B), (C), and (D) are incorrect reactions.
Explanation of Incorrectness:
- (A) Azo coupling with deactivated aromatic rings like benzoic acid is not favorable, and coupling at the para position to COOH is not expected.
- (B) Acetylation of 4-aminophenol with acetic anhydride primarily occurs at the more nucleophilic amino group, not the hydroxyl group.
- (C) The reaction sequence involving SN2 reaction leads to inversion of configuration at the chiral center, but the product shown has the same configuration as the starting material.
- (D) Hofmann elimination follows the Hofmann rule, yielding the less substituted alkene, whereas the product shown is the more substituted alkene (Saytzeff product).
