Question
Question: Among the following, reaction(s) which give(s) tert-butyl benzene as the major product is (are): A...
Among the following, reaction(s) which give(s) tert-butyl benzene as the major product is (are):
A. 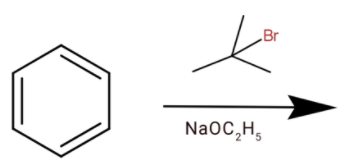
B. 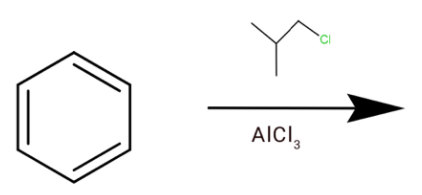
C. 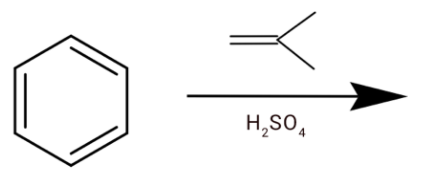
D. 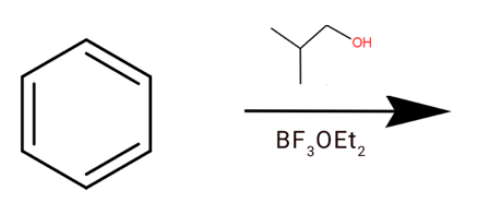
Solution
We need a tertiary alkane which has a less hydrogen atom at the center of it can act as Lewis base i.e., the tert-butyl cation This can lead to formation of the tert-butylbenzene.
Complete step by step solution:
In order to get the major product as tert-butylbenzene, we have to first produce the tert-butyl which will react with benzene which can give us tert-butylbenzene.
(a) Here we have sodium ethoxide and tert butyl bromide. They will react to the elimination reaction and remove the bromide group from the compound. Then we will get
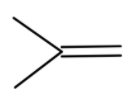
The compound formed will not react with benzene, so we cannot get tert-butylbenzene.
(b) Here we have AlCl3which is a Lewis acid and we have chlorine which is Lewis base. The chlorine will attack AlCl3, which will form a cation.
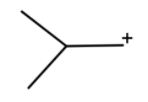
This will go hydride shift to form tert-butyl cation.
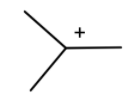
Which can react with a benzene ring, where one hydrogen is removed and the cation is attached to it to form tert-butylbenzene.
Hence option (b) is correct.
(c) We have an alkene and sulphuric acid. The H2SO4 will react with alkene and H+ gets attached to the alkene.
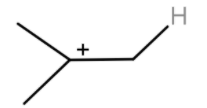
After the hydride shift we will get tert-butyl cation. Which will react with a benzene ring to give tert-butylbenzene.
Hence option (c) is correct.
(d) We have given BF3 in presence of ether. So is generally a gas so to trap it we use ether. So BF3 acts as Lewis acid and oxygen act as Lewis base. This oxygen acts the BF3 and we will get
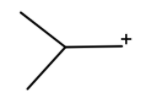
After the hydride shift we will get tert-butyl cation. Which will react with a benzene ring to give tert-butylbenzene.
**Hence option (d) is correct.
Note:**
The hydride shift happens because the tertiary cations are stronger as it involves maximum +I effect and it has maximum hyperconjugation (9 alpha hydrogens). Thus, we can say that tertiary carbocations are more stable than benzyl carbocation.
