Question
Question: Among the following free radical bromination reactions, select those in which \( {2^0} \) halide is ...
Among the following free radical bromination reactions, select those in which 20 halide is the major product.
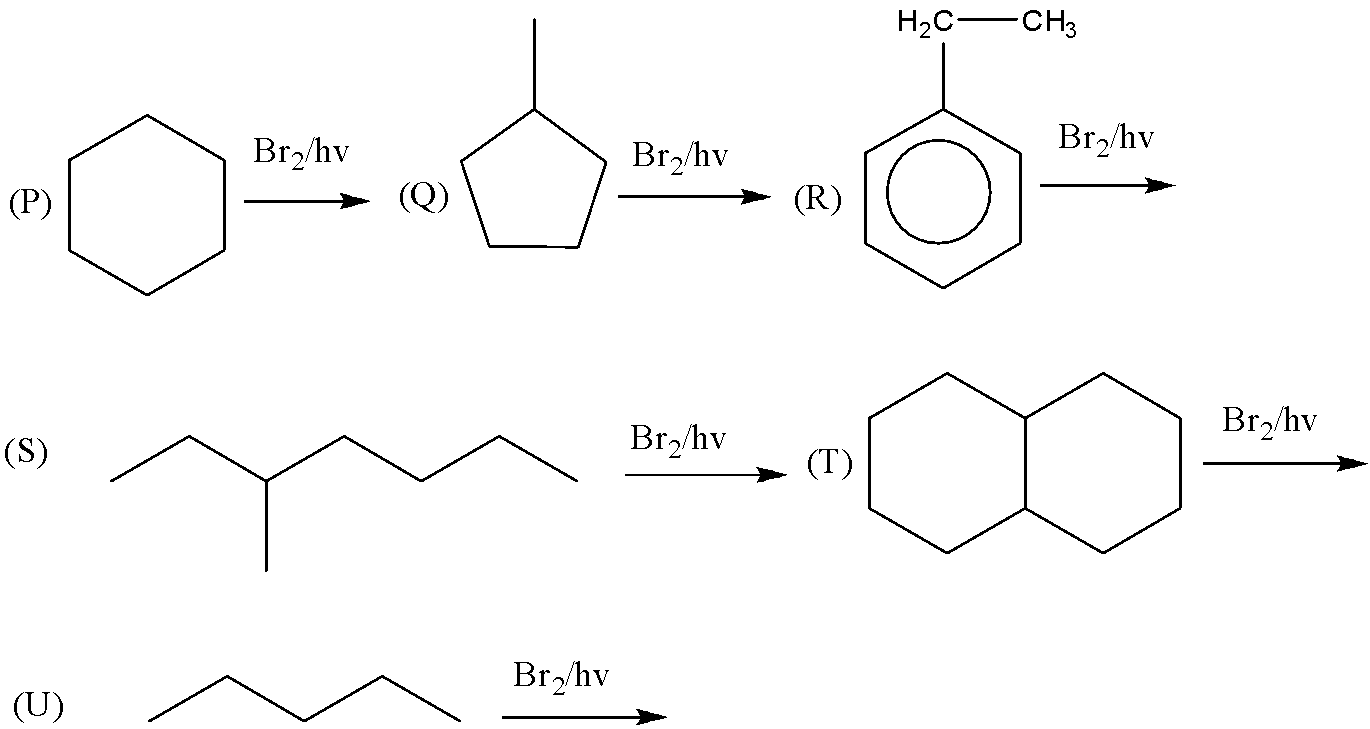
(A) P,Q,R,S
(B) P,R,U
(C) P,R,S,T
(D) P,Q,R,S,T
Solution
Here, we have given the free radical bromination reactions in which we will find the major product. Here we will find the reaction which forms 20 halide as the major product. Bromine is selective; it may form primary, secondary, or tertiary halide.
Complete step by step solution:
First, we will discuss the free radical bromination reactions. Free radical bromination reactions are the halogenation reactions that substitute chlorine or bromine for hydrogen on an alkane. Free radical halogenation reactions are performed in the presence of UV light. Now we will consider some reactions one by one which gives 20 halide as the major product. The 20 halide is the product formed when the carbon atom with the halogen group or any other group is attached with two more carbon atoms. So the reactions are as follows.
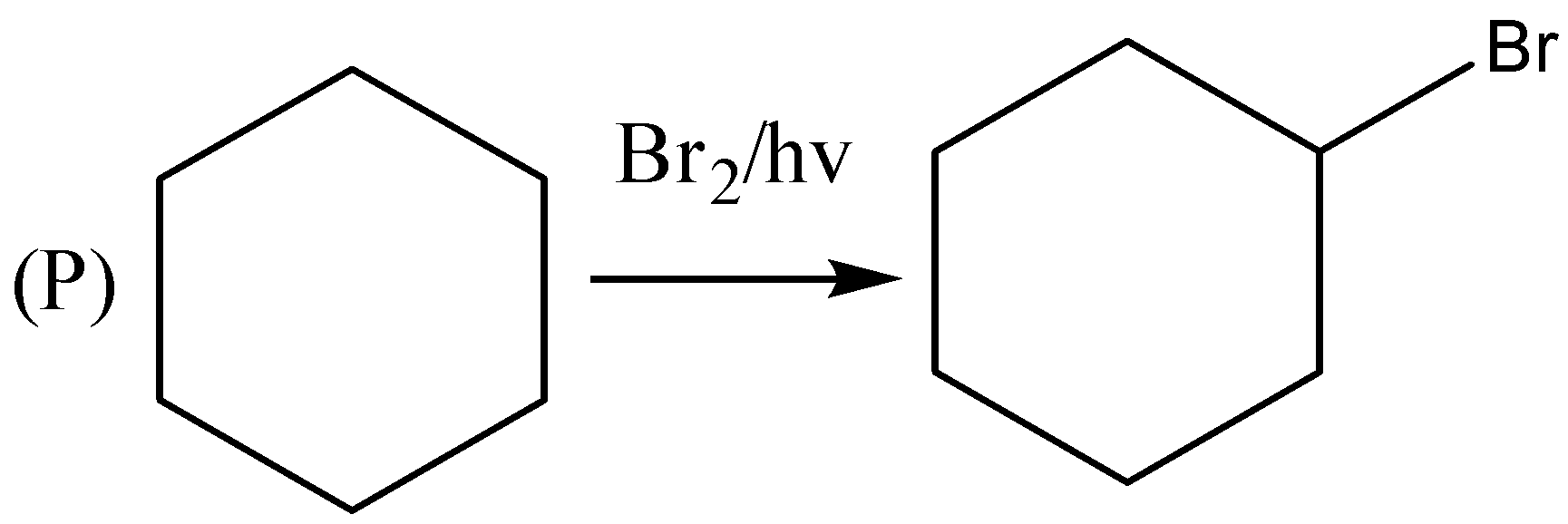
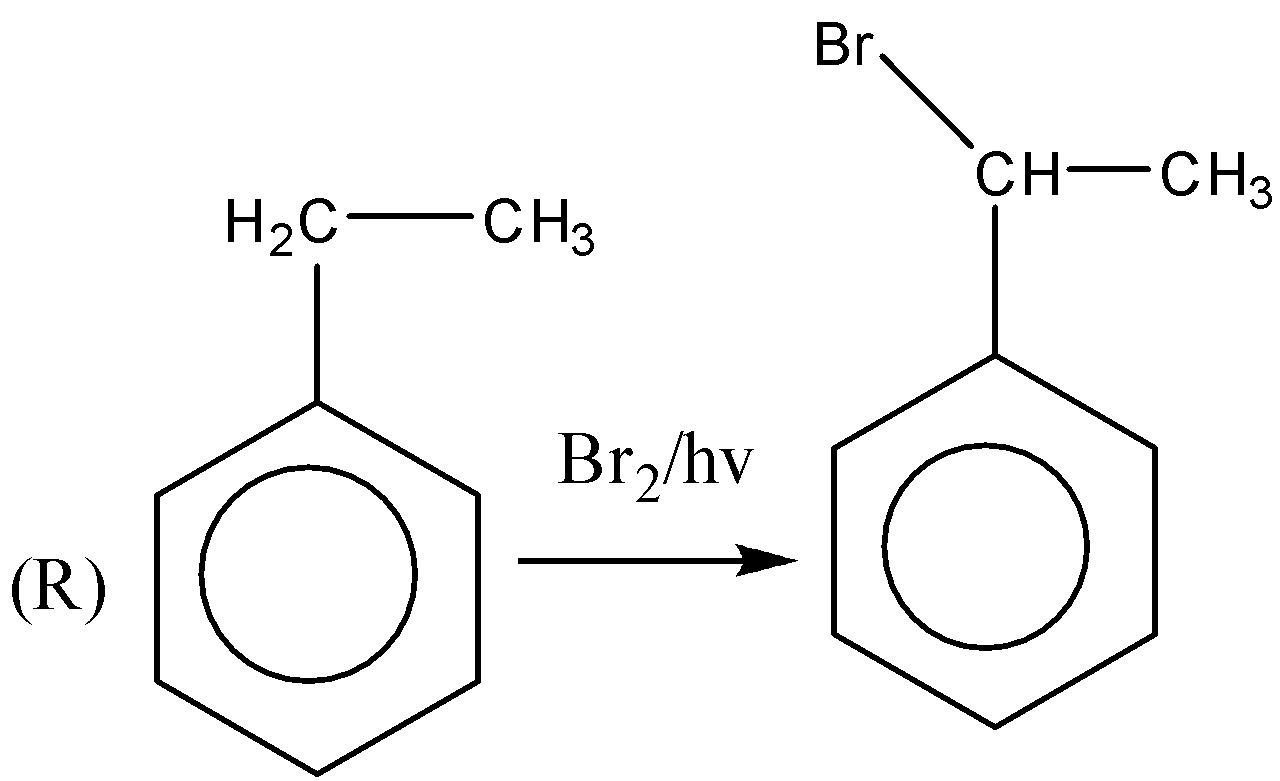
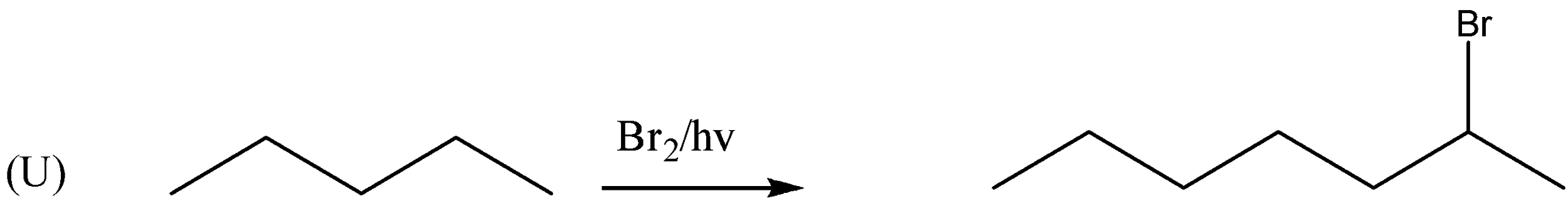
If we observe the major products formed by the reactions P,R,U . We can conclude that all the major products formed are 20 halide or secondary halide product. The carbon attached with bromine is surrounded by two carbon atoms. Therefore, the major product is 20 a halide. Similarly, (R) the carbon attached with bromine is attached with two carbon atoms. Therefore, the major product is 20 a halide. In reaction (U) also the major product formed is 20 a halide. In the reactions, Q,S,T the major products formed are 30 or tertiary halides.
Therefore, the correct option is (B).
Note:
Bromination is slower and more selective because a bromine atom is a less reactive hydrogen atom abstraction agent than a chlorine atom as reflected by the greater bond energy of HCl than HBr .
