Question
Question: Among the following compounds, the strongest acid is: A. \({\text{HC}} \equiv {\text{CH}}\) B. \...
Among the following compounds, the strongest acid is:
A. HC≡CH
B. C6H6
C. C2H6
D. CH3OH
Solution
The species which donates hydrogen ions when dissolved in water is known as an acid. The acidic nature of a substance is directly proportional to the electronegativity of the groups attached. All the hydrogens are not acidic in nature. The hydrogen attached with electronegative atoms is acidic. The hydrogen atom attached to a carbon atom cannot be acidic unless some stabilizing factor balances the negative charge after hydrogen removal.
Complete step by step solution:
We are given four compounds acetylene (HC≡CH), benzene (C6H6), ethane (C2H6) and methanol (CH3OH).
The structures of acetylene, benzene, ethane and methanol are as follows:
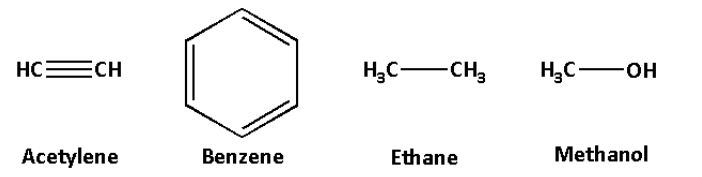
In the compounds, acetylene, benzene and ethane, the carbon atom is attached to another carbon atom.
In methanol, the carbon atom is attached to the oxygen atom.
The electronegativity of oxygen is higher than the electronegativity of the carbon. Thus, methanol is more electronegative than acetylene, benzene and ethane. Electronegativity is the tendency of an atom to attract a bonding pair of electrons.
Thus, the strongest acid is CH3OH.
**Thus, the correct option is (D) CH3OH.
Note: **
We can also determine the acidity of the given compounds by determining the s-character of the compound and the positive inductive effect of the group. The s-character refers to the contribution of the sigma bond in the compound. The higher the s-character more acidic is the compound. The sp hybridised carbon atom is more acidic. The carbon atom of acetylene is sp hybridised but the electronegativity of the oxygen atom of methanol is more and thus, methanol is more acidic.
