Question
Question: Among the following Arenium ions which one is more stable? 
Solution
The stability of the arenium ions can be understood and explained with the help of inductive and resonance effect.
Step by step answer: In organic chemistry, electron displacement effects such as inductive effect and resonance effect play a very significant role and can help us in deducing or explaining different properties or behavior of the species they are present in.
We can understand the inductive effect as polarization of a σ− bond causing polarization of the adjacent σ− bond(s). We know that polarization of a bond occurs when there is a displacement due to the presence of an atom or group. Based on this, we can have electron withdrawing groups or electron donating groups. For example, −NO2,−CN are electron withdrawing groups whereas −CH3,−C2H5 are electron donating groups.
Now, for resonance effect, we can understand this as polarization in a molecule due to the interaction of two adjacent π− bonds or one π− bond with a lone pair of electrons. It can be of two types:
+R effect: in this case, we observe electron displacement away from the atom/group increasing electron density at certain positions in the molecule. Examples include −NH2and−OH
−R effect: in this case, we observe electron displacement towards the atom/group decreasing electron density at certain positions in the molecule. Examples include −NO2and−CN
Now, in the light of above discussion let’s have a look at the given arenium ions which are basically σ− complex formed by electrophilic attack on an arene. Here, we have benzene attacked by E+ electrophile which can be shown as:
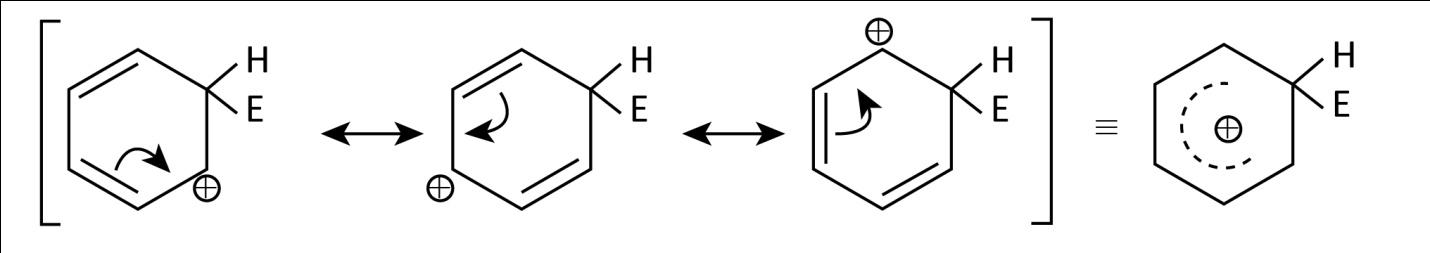
As we can see that it is a resonance stabilized ion.
Now, when a nitro group is present at the benzene which is an electron withdrawing group and shows −R effect due to which electron density is decreased making substitution more difficult and arenium ion less stable.
Hence, the correct option is A
Note: substitution reactions can take place in nitrobenzene as well but at meta- position not at ortho- or para- as electron density is decreased even lesser there than at meta- position
