Question
Question: Among \({\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}\), \({{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5...
Among Me3N, C5H5N and MeCN (Me=methyl group) the electronegativity of N is in the order of:
A) MeCN>C5H5N>Me3N
B) C5H5N>Me3N>MeCN
C) Me3N>MeCN>C5H5N
D) Electronegativity is the same in all.
Solution
Electronegativity of an atom depends on its state of hybridisation. Determine the hybridisation of nitrogen i.e. N in each of the given compounds. From the hybridisation, calculate the percent s-character and then decide the most electronegative carbon atom.
Complete step-by-step answer:
We are given three compounds Me3N, C5H5N and MeCN (Me=methyl group). Let us calculate the percent s-character for nitrogen atom in each of the given compounds.
Consider the compound Me3N:
The structure of the compound Me3N is as follows:
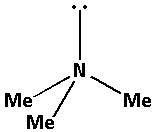
The nitrogen atom in Me3N forms three bond pairs and also there is one lone pair of electron. Thus, Me3N has a total four bond pairs. Thus, the nitrogen atom in Me3N is sp3 hybridised.
Percentage s-character in sp3 hybridised orbital =41=0.25×100%=25%.
Thus, the percentage s-character of the nitrogen atom in Me3N is 25%.
Consider the compound C5H5N:
The structure of the compound C5H5N is as follows:
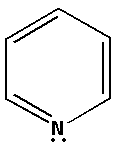
The nitrogen atom in C5H5N forms two bond pairs and also there is one lone pair of electron. Thus, C5H5N has total three bond pairs. Thus, the nitrogen atom in C5H5N is sp2 hybridised.
Percentage s-character in sp2 hybridised orbital =31=0.33×100%=33%.
Thus, the percentage s-character of the nitrogen atom in C5H5N is 33%.
Consider the compound MeCN:
The structure of the compound MeCN is as follows:
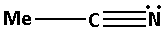
The nitrogen atom in MeCN forms one bond pair and also there is one lone pair of electrons. Thus, C5H5N has total two bond pairs. Thus, the nitrogen atom in MeCN is sp hybridised.
Percentage s-character in sp hybridised orbital =21=0.5×100%=50%.
Thus, the percentage s-character of the nitrogen atom in MeCN is 50%.
We know that more the s-character, more is the electronegativity of the nitrogen atom.
Thus, the electronegativity of N is in the order of MeCN>C5H5N>Me3N.
Thus, the correct option is (A) MeCN>C5H5N>Me3N.
Note: Remember that more the s-character, more is the electronegativity of the nitrogen atom. Thus, the sp hybridised nitrogen atom is most electronegative than the sp2 hybridised nitrogen atom which is more electronegative than the sp3 hybridised nitrogen atom
