Question
Question: Among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H...
Among H2O, H2S,H2Se & H2Te the one with highest boiling point is:
A ) H2O because of hydrogen bonding interactions.
B ) H2Te because of its higher molecular weight.
C ) H2S because of hydrogen bonding interactions.
D ) H2Se because of its lower molecular weight.
Solution
A hydrogen bond is formed when hydrogen atom is attached to electronegative nitrogen, oxygen or fluorine atom. Due to formation of hydrogen bonds, molecules associated and more energy are needed to break these associations. This results in higher boiling. With increase in the molecular weight, the boiling point also increases. But the effect of hydrogen bonding is more pronounced than the effect of increase in the molecular weight.
Complete answer:
Among H2O, H2S,H2Se & H2Te the one that can form intermolecular hydrogen bonds is H2O. This is because in H2O molecules, a hydrogen atom is attached to an electronegative oxygen atom.
The hydrogen bonding in water molecules is as shown below:
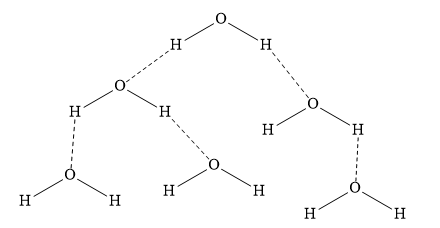
In the above diagram, the dotted line represents intermolecular hydrogen bonds.
H2S,H2Se & H2Te cannot form intermolecular hydrogen bonds as in these compounds, hydrogen atom is not attached to electronegative nitrogen, oxygen or fluorine atom.
Among H2O, H2S,H2Se & H2Te the one with highest boiling point is H2O.
Hence, the correct answer is the option A ).
Additional Information: In the oxygen family, the atomic mass increases in the order oxygen < sulfur < selenium < tellurium. On moving down the group the atomic mass increases. With increase in the atomic mass of the element, the molecular weight of its hydride also increases.
With increase in the molecular weight, the boiling point should also increase. But the effect of hydrogen bonding on the boiling point outweighs the effect of increase in the molecular weight.
Note: Do not confuse between option A) and B) because of higher molecular weight of H2Te. The effect of hydrogen bonding on the boiling point outweighs the effect of increase in the molecular weight. Based on the combined effects of hydrogen bonding and the increase in the molecular weight, you can conclude that among H2O, H2S,H2Se & H2Te , hydrogen sulfide H2S has lowest boiling point as it has lowest molecular weight among H2S,H2Se & H2Te and it cannot form hydrogen bonds.
