Question
Question: Among \({N_2}O,S{O_2},I_3^ + ,I_3^ - \) , the linear species are \({N_2}O\) and \(I_3^ - \) . A.Tr...
Among N2O,SO2,I3+,I3− , the linear species are N2O and I3− .
A.True
B.False
Solution
Linear species means the structure of the given compound is linear. It is aligned horizontally. Every molecule has different shape and structure depending upon the number of lone pairs and the type of bonding it has.
Complete step by step answer:
1.N2O
Nitrous oxide is also known as the laughing gas.
It is a non flammable gas at room temperature.
It is slightly metallic in taste and smell.
It is heavier than air.
The lewis structure of nitrous oxide is as follows:
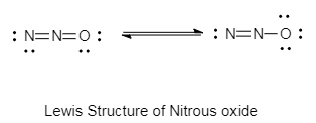
In the diagram given above, the type of bonding in nitrous oxide is covalent bonding.
Due to the presence of sigma bonds and absence of lone pairs on the central atom, the stable structure of nitrous acid is linear.
2.SO2
It is also known as sulphur dioxide.
The sulphur dioxide gas is toxic.
The smell of the burnt match sticks is of the sulphur dioxide.
It is a covalent compound.
The structure of sulphur dioxide is as follows:
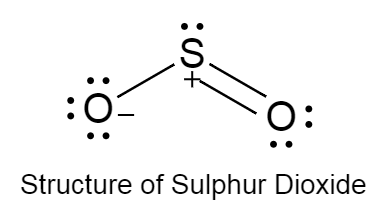
The structure of sulphur dioxide is bent because due to the high electronegativity of oxygen it tries to pull sulphur atom towards itself.
3.I3+
The structure of I3+ is given below as follows:
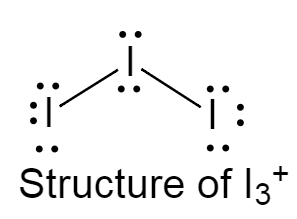
It has a bent structure because of the presence of two lone pairs of electrons on the central iodine atom.
4.I3−
The structure of I3− is as follows:
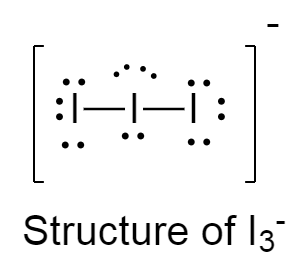
There are three lone pairs on the central iodine atom because of which the adjacent iodine atoms try to repel from each other as much as possible at a greater distance causing the angle to be 180∘ .
Because of this repulsion, The structure of I3− is linear.
Since the structure of I3−and N2O is linear, they are linear species.
So the correct answer is option A) True.
Note: I3− is neither polar or nonpolar molecule. As it is soluble in water it is known as a polar molecule. I3− has more number of lone pairs as compared to I3+ . The smell of sulphur dioxide gas is not at all tolerable.
