Question
Question: Among \[K{O_2}\],\[Al{O_2}^ - \],\[Ba{O_2}\]and \[N{O_2}^ + \] unpaired electron is present in: A....
Among KO2,AlO2−,BaO2and NO2+ unpaired electron is present in:
A.NO2+ and BaO2
B.KO2 and AlO2−
C.KO2 only
D.BaO2 only
Solution
We must know that molecules only have unpaired electrons and not for an individual atom. Therefore, we just complete the valency of each atom in a molecule. If any electrons are left, they are unpaired. Also the molecules are ionic in nature. So we have to draw the possible Lewis structure and check for possible unpaired electrons.
Complete step by step answer:
Let’s start with discussing a little bit about the electron dot structure (Lewis structures) that we are going to use for solving this problem. Electron dot structures are the diagrams which tell us about the bonded and unbonded electron, paired and unpaired electron with the help of dots around the atom symbol, each dot represents an electron.
For drawing the electron dot structure the student should follow the following steps:
Find the total number of valence electrons. For example, O2 has a total number of 6+6=12 electrons.
If it’s a molecule with more than 2 atoms then put the least negative atom in the centre. For example, in NO2 you will keep the nitrogen (N) between the two oxygen (O) i.e.O−N−O.
Put two electrons in between the atoms to form the bond. For example, in HCl you will keep H:Cl like this a bond between hydrogen (H) and chlorine (Cl) has been formed.
After forming the bond you will complete the octets on the outside atoms. For example, in case of HCl after H:Cl you will complete the octet of Cl− like this the octet of Cl is complete and the Lewis dot structure is being made.
But if central atom does not have an octet then move the electrons from outside to the inside i.e. in case of O2 from 4th point we get this shows that one of the O is not having complete octet only 6 electron so we move one electron pair from outside to the inside and we get like this both the atoms have a complete octet.
So, let’s begin drawing the electron dot structure for each molecule given to us
Case 1
The electron dot structure of KO2 is
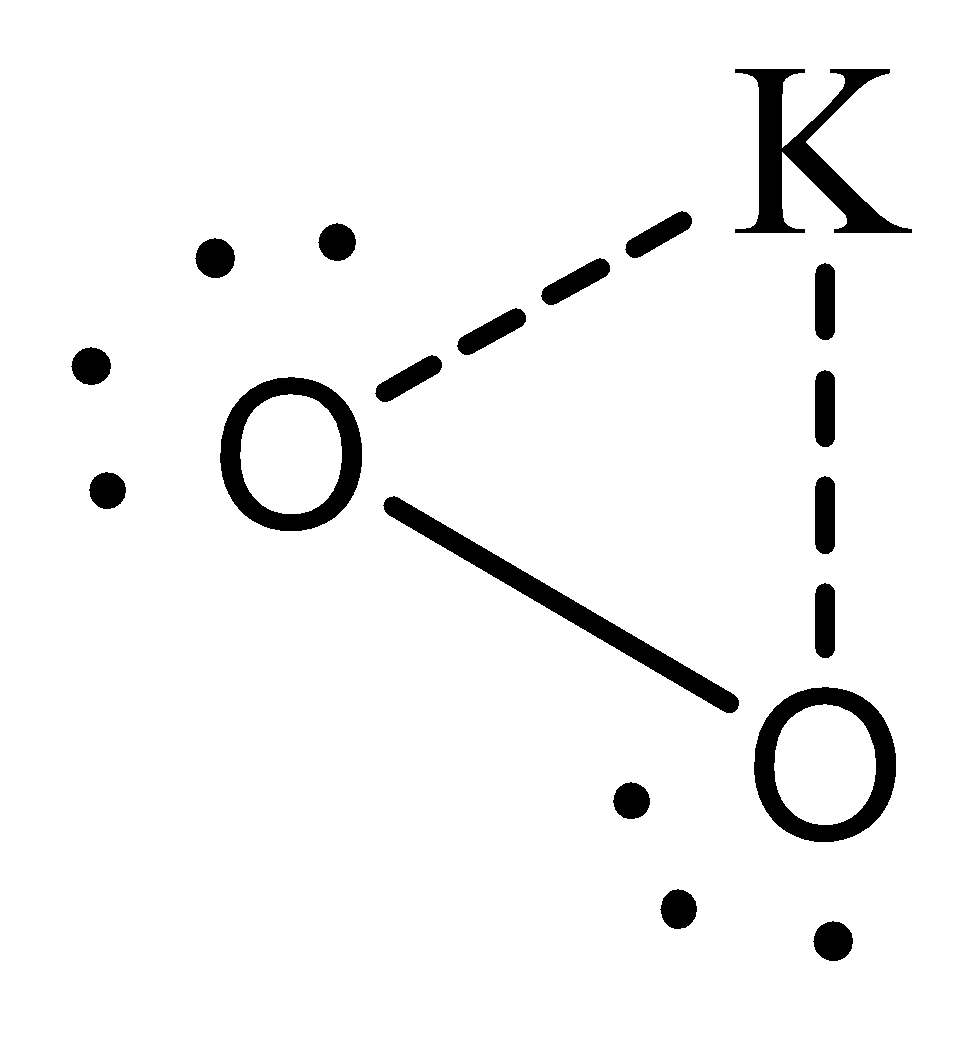
The O2 is a superoxide present in this and the given is the lewis structure for the same. Clearly there is one unpaired electron in this.
Case 2
AlO2− is an ionic compound, the oxygen atom requires 2 electron for completing its octet and Aluminium needs to release 3 electron to achieve noble gas configuration, So, the aluminium (Al) gives two electrons to first oxygen, The octed of first oxygen is complete and after that Al gives the last electron the second oxygen and itself achieves noble gas configuration, Now the second electron gets one more electron from outside and completes its octet. Hence there is no unpaired electron in this.
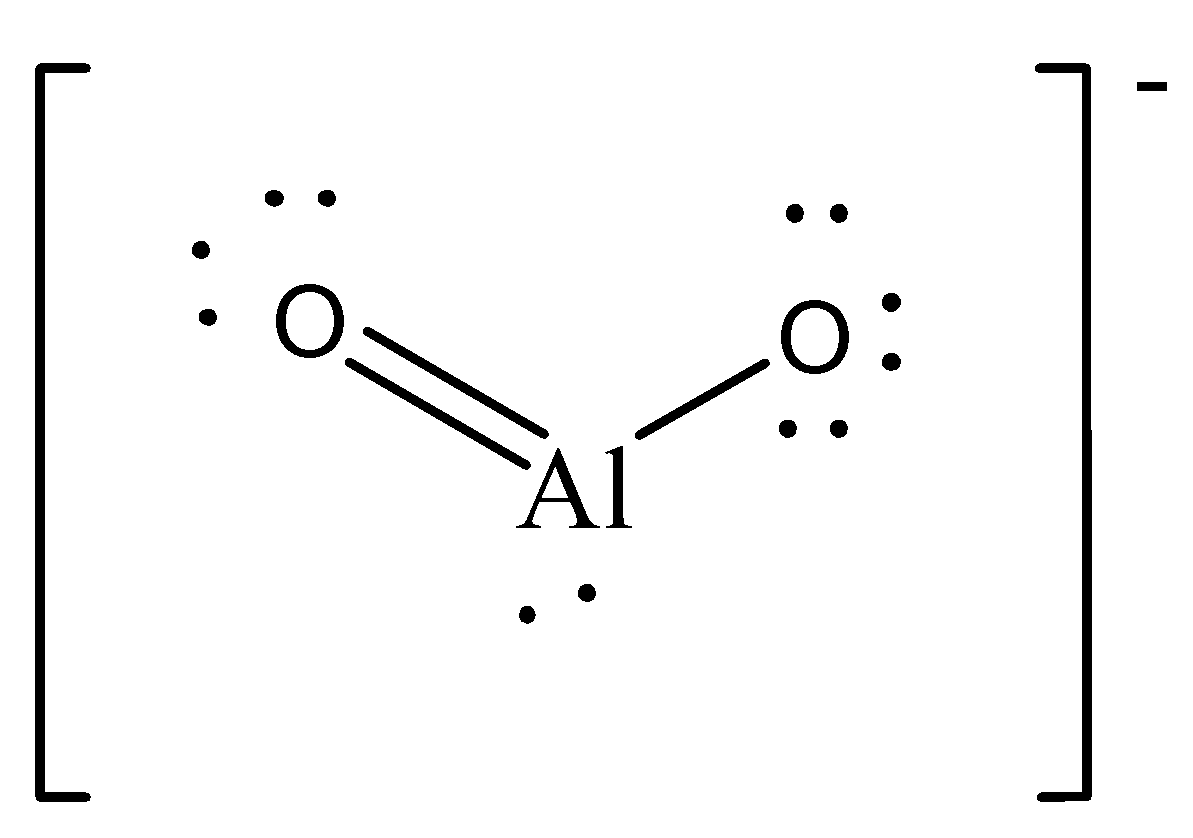
Case 3
In case of BaO2the O22−present is a superoxide and barium (Ba) is having the ionic state of Ba2+so a ionic bond is being formed and no unpaired electron is present
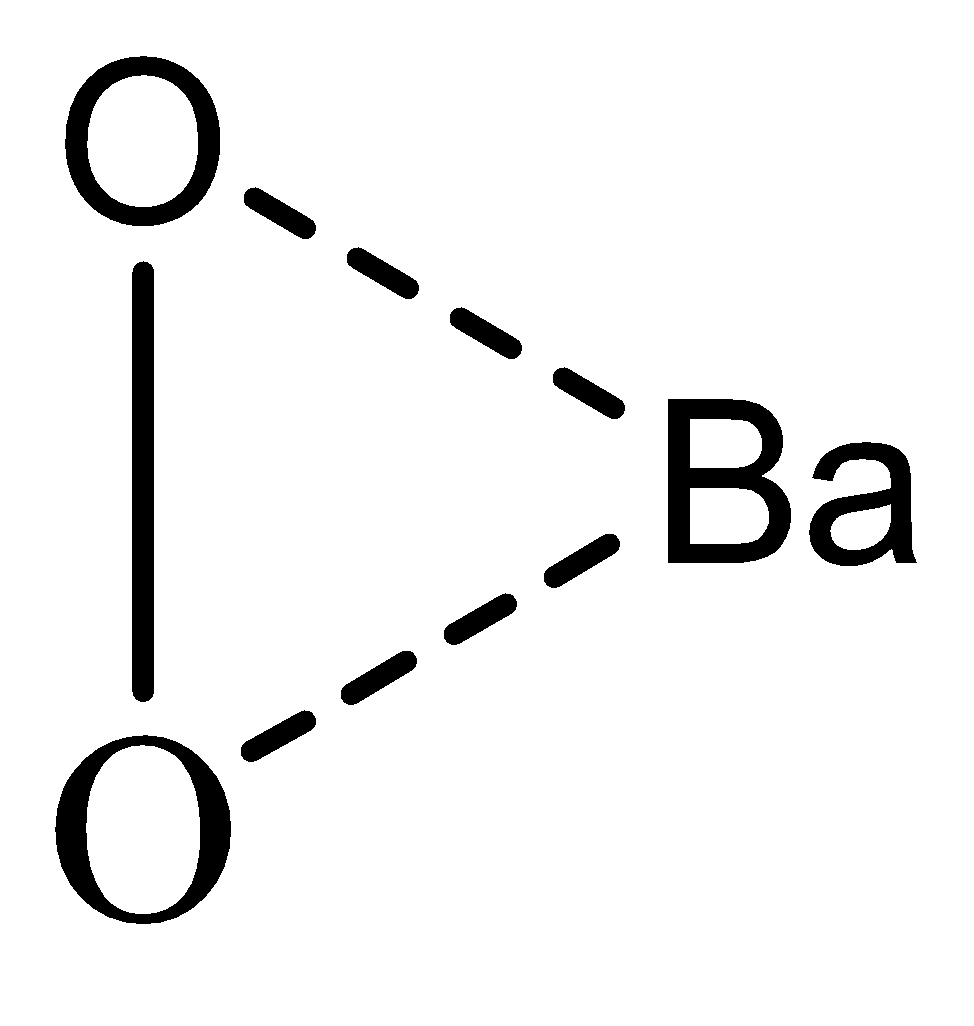
Case 4
The electron dot structure for NO2+ is
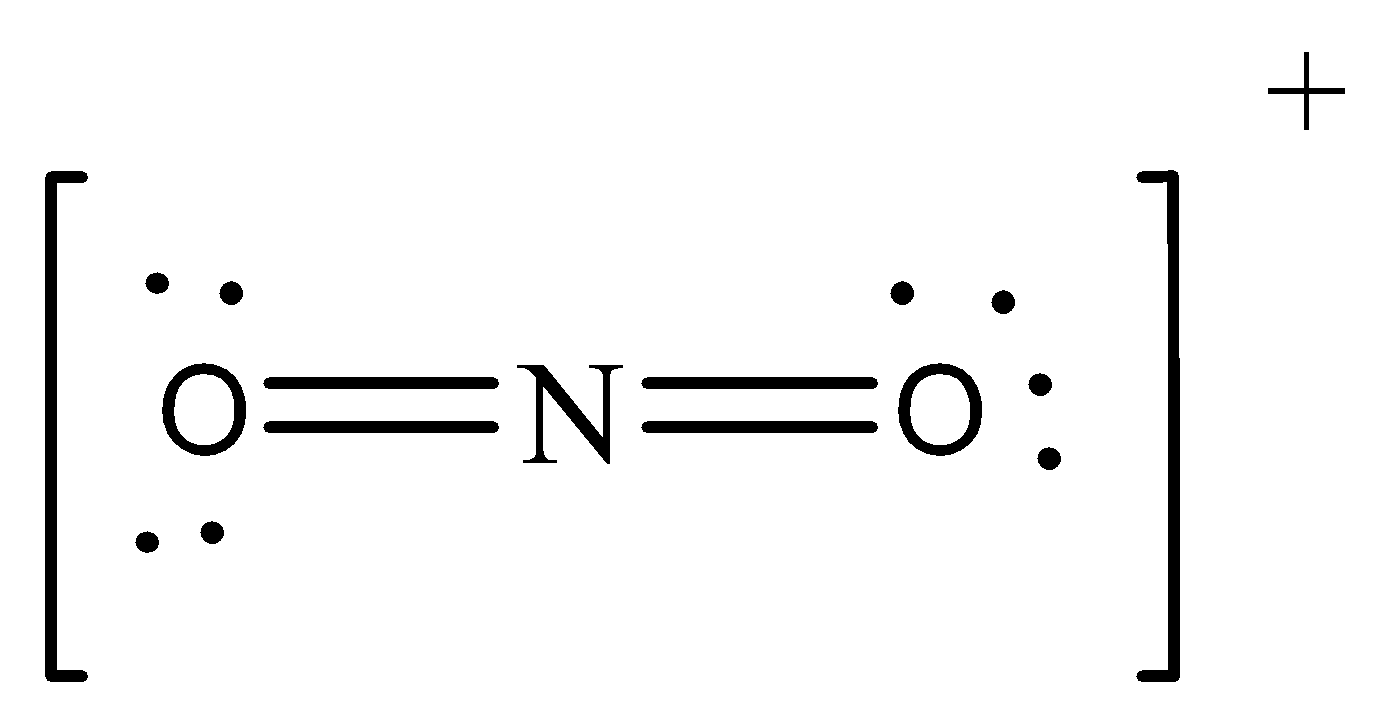
Clearly no unpaired electron is here.
So, the correct answer is Option C .
Note:
We must know that the ionic molecules which don't form covalent bonds are important to check how they are sharing the electron. You should know their stable oxidative state and use that to come to a conclusion. Like molecule Al2O3 is an ionic molecule and we can clearly see that Al oxidation state is +3 and oxygen is -2 so 2×3 = 3×2 hence the molecule is not having any unpaired electron. We can use this approach as well.
