Question
Question: Among \[HCl\,,\,{H_2}O\,\] and \[N{H_3}\] ,which has a higher boiling point and why?...
Among HCl,H2O and NH3 ,which has a higher boiling point and why?
Solution
The boiling point also referred to as B.P is a temperature at which the vapour pressure of the liquid equals the external pressure surrounding the liquid. The boiling point (B.P) of a liquid depends upon the atmospheric pressure. The factor that influences the B.P is the strength of intermolecular forces., like ionic bonding, hydrogen bonding, etc.
Complete step by step answer:
The electrostatic force of attraction between the Hydrogen and the electronegative atom such as nitrogen, oxygen, fluorine, etc., is called Hydrogen bonding. In such bonds, the hydrogen acts as a donor and the electronegative element acts as an acceptor. The Hydrogen bond is having a special type of interaction i.e., dipole-dipole interaction.
Hydrogen bond strength ranges from 4kJ - 50kJmol−1 of hydrogen bonds.
From HCl,H2O and NH3 ,
H2O is having the highest boiling point.
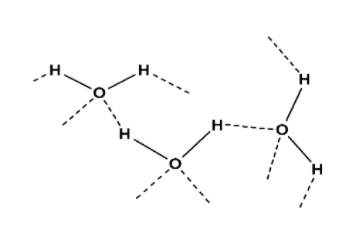
The H2O is hydrogen bonding. But even in NH3 there is hydrogen bonding, since it's weaker than H2O due to the less electronegativity of nitrogen than oxygen.
The main reason H2O is having great hydrogen bond because H2O is having bent shape structure that allows for the greater interaction with the adjacent H2O molecules.
The H2O is having more hydrogen bonding than HF . Even though the Fluorine is having higher electronegativity than Oxygen, the important thing to be noted is that HF can form two hydrogen bonding whereas, H2O can form four hydrogen bonding. Due to this reason, HF is having less boiling point than H2O.
When we see the HCl , it lacks the ability to form hydrogen bonding. This is due to the size of Cl that hides the hydrogen to form intermolecular hydrogen bonding. Since hydrogen is very small.
H2O>NH3>HCl
Note: In H2O, Oxygen has two lone-pairs. In NH3 , nitrogen has one lone-pair. So, H2O form four H−bonds whereas, in the case of NH3 ,it forms two H−bonds. In the case of HCl we saw that chlorine hides the hydrogen due to their sizes. That’s the reason, H2O is more stable and it needs more energy to break the bond. As the hydrogen bond increases the compound is having more boiling point.
