Question
Question: Among \(Ca{H_2},N{H_3},NaH\) and \({B_2}{H_6}\), which are covalent hydrides? A) \(N{H_3}\) and \(...
Among CaH2,NH3,NaH and B2H6, which are covalent hydrides?
A) NH3 and B2H6
B) NaH and CaH2
C) NaH and NH3
D) CaH2 and B2H6
Solution
A compound will be covalent or ionic is based on the oxidation state of the central atom present in the compound if it exceeds a predefined value the compound is covalent else it is ionic in nature. All the metal hydrides of group one of periodic table are ionic in nature due to high electro-positive nature of alkali metals.
Complete step by step answer:
As we know that sodium (Na) belongs to group one of the periodic table and is an alkali metal which is highly electropositive in nature hence it will always form ionic compound thus a hydride of sodium will also be ionic in nature hence NaH is ionic in nature
Also if we find the oxidation state of sodium is +1 which is very low that way also it is ionic in nature
Calcium (Ca) belongs to group two of the periodic table and is an alkaline earth metal even though it is less electropositive than sodium but still has tendency to form only ionic bond which means CaH2 is also Ionic in nature
Just like sodium, calcium also exist in a very low oxidation state of +2 in CaH2 and that also makes it an Ionic compound
Now coming to NH3 we can see in the following image that the central atom is nitrogen which is highly electronegative in nature hence it generally forms covalent bond also there is not much net electronegativity difference between N and H that also confirms its covalent nature
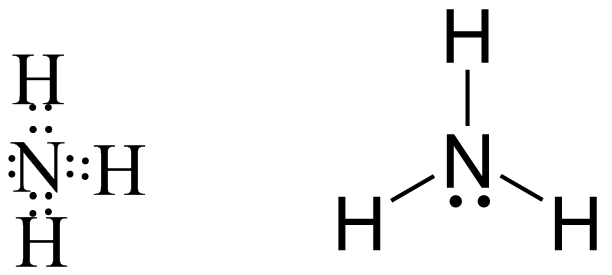
Finally coming to B2H6 in which central atom is Boron which is belongs to group 13 of periodic table shows completely different behavior from the other group member because of its anomalous nature
Forms a three centered two electron covalent bond in B2H6
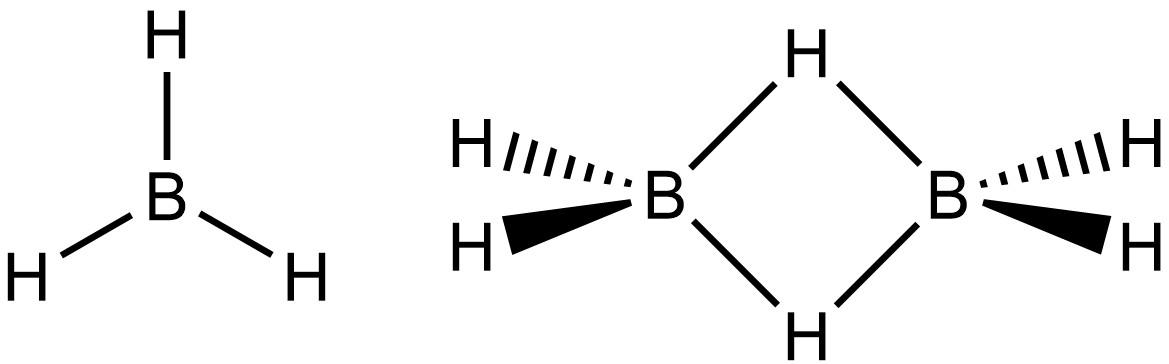
Borane Diborane
Thus from the above four hydrides only two that are B2H6 and NH3 are covalent in nature
Making option ‘A’ as the correct option.
Note:
The special type of three centered two electron covalent bond in B2H6 is known as Banana bond and is asked in various competitive exams so do remember that Diborane (B2H6) is an example of a compound forming banana bond.
