Question
Question: Among \({{B}_{2}}{{H}_{6}},{{B}_{3}}{{N}_{3}}{{H}_{6}},{{N}_{2}}O,{{N}_{2}}{{O}_{4}},{{H}_{2}}{{S}_{...
Among B2H6,B3N3H6,N2O,N2O4,H2S2O3 and H2S2O8, the total number of molecules containing covalent bond between two atoms of the same kind is ⋯⋯
Solution
A covalent bond is formed by the equal sharing of electrons .The given molecules are diborane, borazine, nitrous oxide, dinitrogen tetroxide, thiosulfuric acid and Peroxydisulfuric acid. By identifying the structures of given compounds, we can find the number of molecules containing covalent bonds between two atoms of the same kind.
Complete step by step solution:
-As we know, a covalent bond is formed by the equal sharing of electrons from both the participating atoms in the bond. Shared pair or bonding pair is the pair of electrons participating in this type of bonding. The covalent bonds are also known as molecular bonds. The Sharing of bonding pairs will guarantee that the atoms achieve stability (octet) in their outer shell similar to the atoms of noble gases.
- Let's take each of the given compounds and look at its structure in order to identify the covalent bond between two atoms of the same kind. The first molecule is diborane B2H6 and its structure is given below
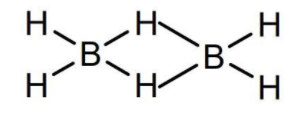
- As we can see there is no covalent bond between two atoms of the same kind (between boron’s).The second molecule is borazine B3N3H6 and its structure is given below
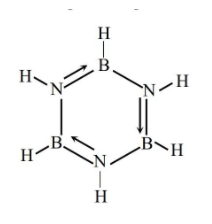 In borazine also, there is no covalent bond between two atoms of the same kind. The third molecule is nitrous oxide N2O and its structure is given below
In borazine also, there is no covalent bond between two atoms of the same kind. The third molecule is nitrous oxide N2O and its structure is given below
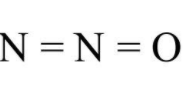
As we can see there is a covalent bond between two nitrogen atoms and hence N2O contains covalent bond between two atoms of the same kind. The fourth molecule is dinitrogen tetroxide N2O4 and its structure is given below
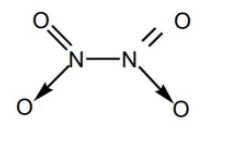
In dinitrogen tetroxide also there is a covalent bond between two nitrogen atoms and hence N2O4 contains covalent bond between two atoms of the same kind. The fifth molecule is H2S2O3 thiosulfuric acid and its structure is given below.
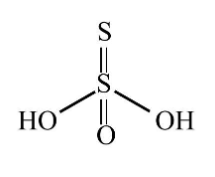
In H2S2O3 there is a covalent bond between two sulfur atoms and hence H2S2O3 contains covalent bond between two atoms of the same kind. The sixth molecule is Peroxydisulfuric acid, H2S2O8and its structure is given below
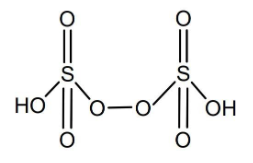
In H2S2O8 there is a covalent bond between two oxygen atoms and hence H2S2O8 contains covalent bond between two atoms of the same kind.
Therefore the molecules N2O,N2O4,H2S2O3 and H2S2O8 contains covalent bond between two atoms of the same kind.
Thus the Total number of molecules containing covalent bonds between two atoms of the same kind are 4.
Note: Keep in mind that according to the number of shared electron pairs, the covalent bond can be classified into Single Covalent Bond, Double Covalent Bond and Triple Covalent Bond. The difference between covalent and ionic bonds should also be noted. A bond between two ions is called ionic bonds while the covalent bonds include pairs of electrons by two atoms binding them in a fixed orientation.
