Question
Question: Out of the following Skeleton structure from below, which of the following requires multiple bond & ...
Out of the following Skeleton structure from below, which of the following requires multiple bond & lone pair both to complete the lewis structure.
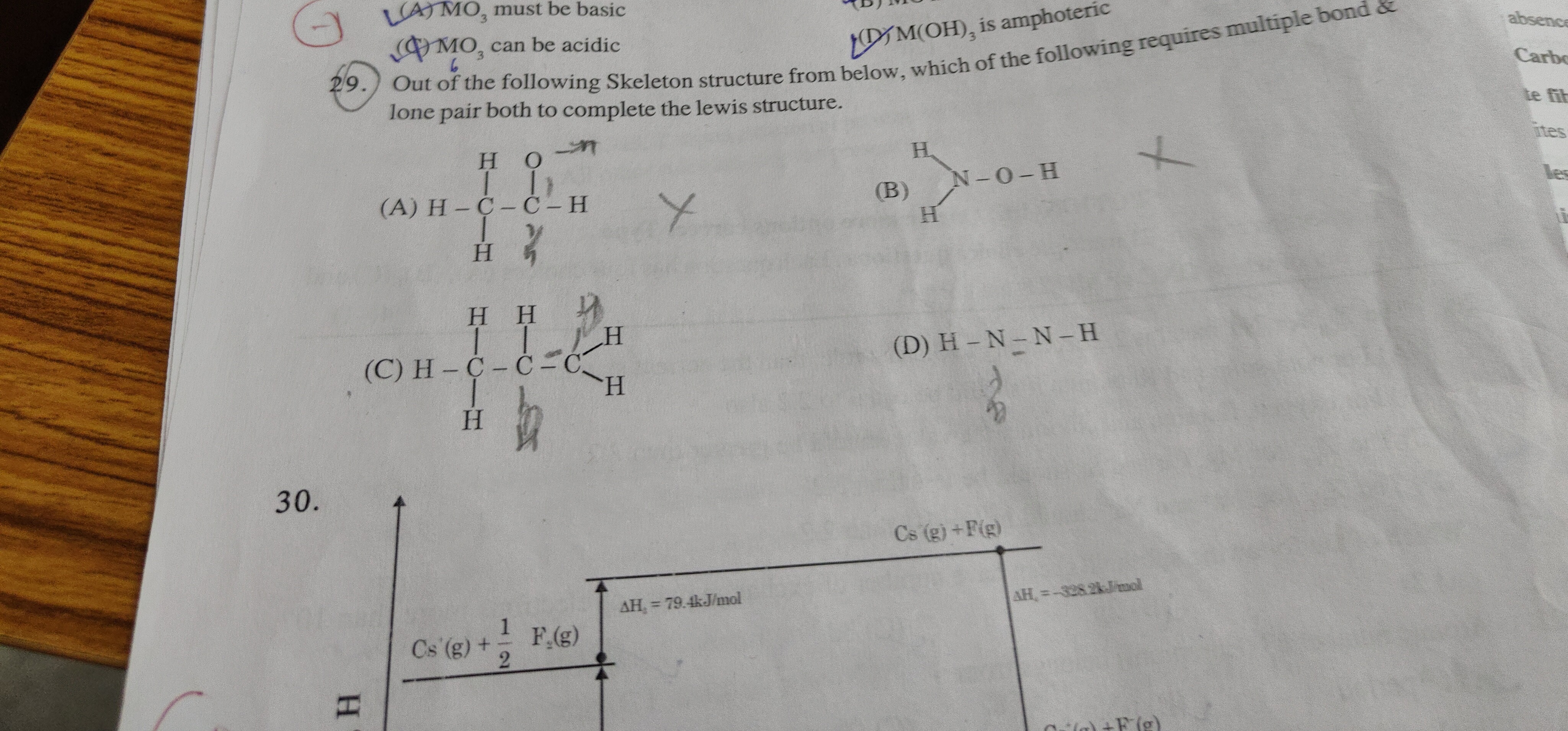
H O
| |
H-C-C-H
| |
H H
H
\
N-O-H
/
H
H H H
| | \
H-C-C-C-H
| | /
H H
H-N-N-H
D
Solution
To determine which skeleton structure requires both multiple bonds and lone pairs to complete its Lewis structure, we need to draw the complete Lewis structure for each given option and check for the presence of both features.
Key principles for drawing Lewis structures:
- Calculate the total number of valence electrons.
- Draw the skeleton structure, connecting atoms with single bonds.
- Distribute the remaining electrons as lone pairs to satisfy the octet rule (or duet for hydrogen) for terminal atoms first, then central atoms.
- If the central atom(s) do not have an octet, convert lone pairs from adjacent atoms into multiple bonds.
Let's analyze each option:
(A) Skeleton: H-C-C-H with H and O attached as shown
The provided skeleton is ambiguous. Let's interpret it as the most common and stable organic molecule that can be formed from C2H4O, which is acetaldehyde (CH₃CHO).
- Total valence electrons: 2(C) + 4(H) + 1(O) = 2(4) + 4(1) + 1(6) = 8 + 4 + 6 = 18 electrons.
- Skeleton and initial bonding: If we arrange it as CH₃CHO, the skeleton is H₃C-CHO. This involves 3 C-H single bonds, 1 C-C single bond, 1 C-H single bond, and 1 C=O double bond.
H
|
H-C - C = O
| \
H H
- Electrons used in bonds: 5 single bonds (C-H, C-C, C-H) = 5 × 2 = 10 electrons. 1 double bond (C=O) = 1 × 4 = 4 electrons. Total = 14 electrons.
- Remaining electrons: 18 - 14 = 4 electrons.
- Distribute remaining electrons: These 4 electrons are placed as 2 lone pairs on the oxygen atom to complete its octet (O already has 4 electrons from the double bond, needs 4 more).
H
|
H-C - C = O:
| \
H H
- Conclusion for (A) as Acetaldehyde: This structure has a multiple bond (C=O) and lone pairs (on O). So, this interpretation fits the criteria.
If the skeleton in (A) is interpreted as ethanol (CH₃CH₂OH), which is C₂H₆O (not C₂H₅O as implied by the drawing):
- Total valence electrons: 2(C) + 6(H) + 1(O) = 2(4) + 6(1) + 1(6) = 8 + 6 + 6 = 20 electrons.
- Lewis structure:
H H
| |
H-C - C - O - H
| | ..
H H
- Conclusion for (A) as Ethanol: This structure has lone pairs (on O) but no multiple bonds. So, this interpretation does not fit the criteria.
Given the ambiguity, let's proceed to other options assuming the question expects a clear fit.
(B) Skeleton: H₂NOH (Hydroxylamine)
- Total valence electrons: 1(N) + 1(O) + 3(H) = 1(5) + 1(6) + 3(1) = 5 + 6 + 3 = 14 electrons.
- Skeleton and initial bonding: N-O-H and two H's on N. This forms 4 single bonds (2 N-H, 1 N-O, 1 O-H). Electrons used = 4 × 2 = 8 electrons.
- Remaining electrons: 14 - 8 = 6 electrons.
- Distribute remaining electrons:
- Oxygen needs 2 lone pairs (4 electrons) to complete its octet.
- Nitrogen needs 1 lone pair (2 electrons) to complete its octet.
- Total lone pairs needed = 4 + 2 = 6 electrons, which matches the remaining electrons.
- Lewis structure:
H
\
:N - O - H
/ ..
H
- Conclusion for (B): This structure has lone pairs (on N and O) but no multiple bonds. It does not fit the criteria.
(C) Skeleton: C₃H₈ (Propane)
- Total valence electrons: 3(C) + 8(H) = 3(4) + 8(1) = 12 + 8 = 20 electrons.
- Skeleton and initial bonding: H₃C-CH₂-CH₃. This forms 10 single bonds (8 C-H, 2 C-C). Electrons used = 10 × 2 = 20 electrons.
- Remaining electrons: 20 - 20 = 0 electrons.
- Lewis structure:
H H H
| | |
H-C-C-C-H
| | |
H H H
- Conclusion for (C): This structure has no multiple bonds and no lone pairs. It does not fit the criteria.
(D) Skeleton: H-N-N-H (Diimine)
- Total valence electrons: 2(N) + 2(H) = 2(5) + 2(1) = 10 + 2 = 12 electrons.
- Skeleton and initial bonding: H-N-N-H. This forms 3 single bonds (2 N-H, 1 N-N). Electrons used = 3 × 2 = 6 electrons.
- Remaining electrons: 12 - 6 = 6 electrons.
- Distribute remaining electrons:
- Each Nitrogen atom currently has 2 bonds (one to H, one to the other N), meaning 4 electrons around it. To complete an octet, each N needs 4 more electrons.
- We have 6 electrons remaining. First, place 1 lone pair (2 electrons) on each N atom. This uses 2 × 2 = 4 electrons.
- Remaining electrons = 6 - 4 = 2 electrons.
- Now, each N has 2 bonds and 1 lone pair, totaling 6 electrons. They each need 2 more electrons for an octet. The 2 remaining electrons can be used to form an additional bond (a double bond) between the two N atoms.
- Lewis structure:
H - N = N - H
: :
- Conclusion for (D): This structure clearly has both a multiple bond (N=N double bond) and lone pairs (one on each N atom). This fits the criteria perfectly.
Comparing the options, (D) unambiguously requires both multiple bonds and lone pairs to complete its stable Lewis structure. While (A) could be interpreted as acetaldehyde which also fits, the skeleton drawing for (A) is very ambiguous and more naturally suggests a single-bonded structure (like ethanol) which does not fit. (D) is the clearest and most direct answer based on the provided skeleton.
The final answer is D
Explanation of the solution:
To complete the Lewis structure for each skeleton, we count valence electrons, form single bonds, then add lone pairs to satisfy octets. If octets are not met, multiple bonds are formed.
- (A) CH₃CHO (Acetaldehyde): Total 18 valence electrons. Requires a C=O double bond and 2 lone pairs on Oxygen. (Fits criteria)
- (B) H₂NOH (Hydroxylamine): Total 14 valence electrons. Forms only single bonds, with lone pairs on N and O. (No multiple bonds)
- (C) C₃H₈ (Propane): Total 20 valence electrons. Forms only single bonds, with no lone pairs. (No multiple bonds, no lone pairs)
- (D) H-N-N-H (Diimine, N₂H₂): Total 12 valence electrons. Requires an N=N double bond and 1 lone pair on each Nitrogen atom. (Fits criteria)
Since the question asks "which of the following requires multiple bond & lone pair both", and (D) clearly and unambiguously fits this description based on its standard stable Lewis structure, it is the correct answer. The ambiguity in (A)'s drawing makes (D) a better choice.
