Question
Question: Ammonia is used as a refrigerating agent. Why?...
Ammonia is used as a refrigerating agent. Why?
Solution
We know that ammonia is gas of no colour and pungent odour. Its chemical formula is NH3 and it is an inorganic compound. When it is dissolved in water, it forms ammonium hydroxide. It is very dangerous in its concentrated form.
Complete step by step answer:
Let’s understand the use of ammonia as a refrigerating agent. Ammonia in anhydrous form is a clear liquid which boils at −28∘F. In the process of refrigeration, in closed containers a liquid is stored under high pressure. The releasing of pressure causes rapid evaporation of liquid forming an invisible gas or vapor. This rapid evaporation results in the drop down of temperature of the liquid until the reaching the normal boiling point of −28∘F. This effect is also observed in evaporation of water from the skin and which causes a cooling effect. This is the reason for using ammonia as a refrigerating agent.
Additional Information:
The structure of ammonia is,
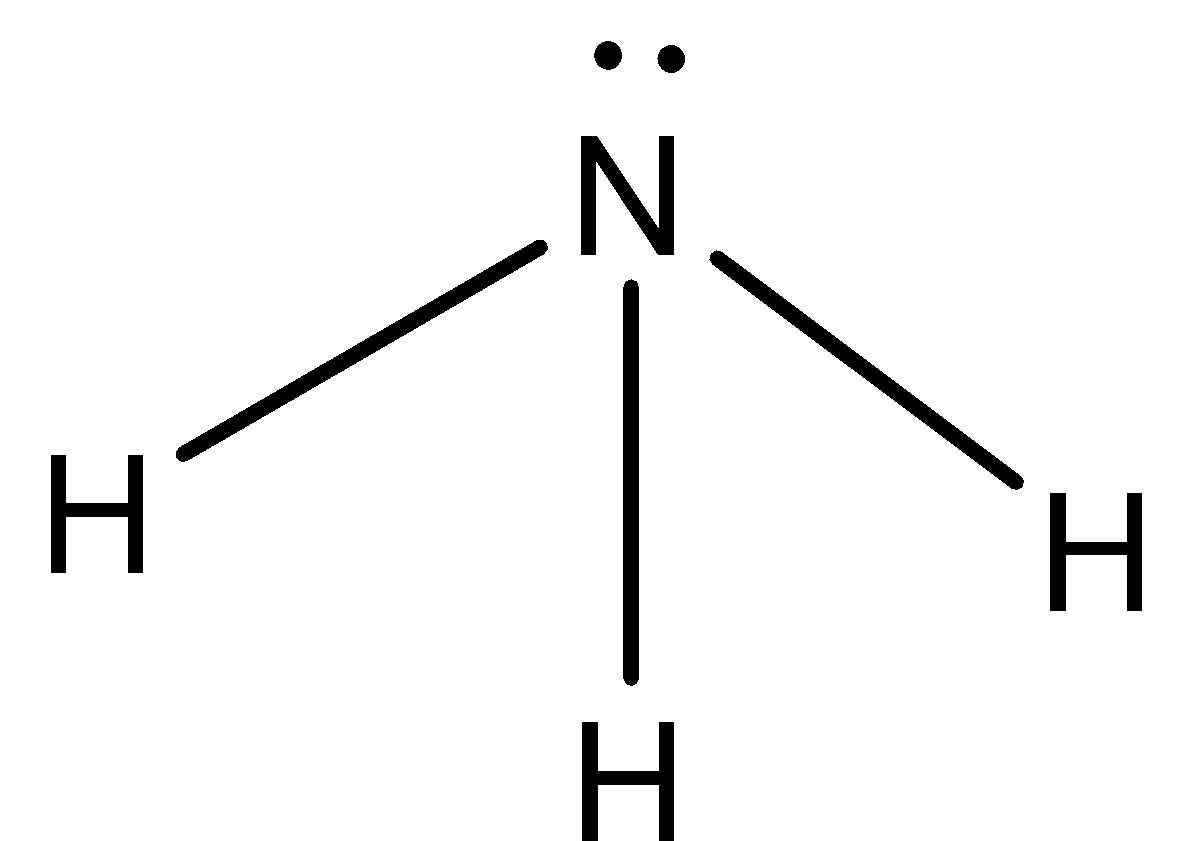
As two lone pairs are present on the nitrogen atom, it can donate its lone pair to form chemical bonds so it acts as a base.
The ammonia is produced in laboratory by heating salt of ammonia such as NH4Cl in presence of strong alkali such as Ca(OH)2.
2NH4Cl+Ca(OH)2→CaCl2+2H2O→2NH3
Another important process is Haber’s process. In this process, hydrogen is reacted with nitrogen to form ammonia.
N2+3H2→2NH3
Note:
There are many uses of ammonia in different fields, such as manufacturing of fertilizers, fermentation industry, and textile industries etc. It is also used as an antimicrobial agent in food products. It is also used in manufacturing of synthetic fibres like nylon and rayon.
