Question
Question: Amine that cannot be prepared by Gabriel Phthalimide synthesis: A. Aniline B. Benzylamine C. M...
Amine that cannot be prepared by Gabriel Phthalimide synthesis:
A. Aniline
B. Benzylamine
C. Methyl amine
D. iso-butylamine
Solution
Gabriel Phthalimide reaction produces only primary aliphatic amine. Aromatic amine cannot be produced in this reaction. Draw the structure of the following compounds and determine which of the following amine is not aliphatic and hence that amine cannot be formed via Gabriel Phthalimide synthesis.
Complete answer:
-Let us understand what is meant by a primary amine. Nitrogen can have three possible bonds because of its trivalent nature. If out of those three bonds, two bonds are occupied by hydrogen, it is called a primary amine. However, If there is only one hydrogen attached to the nitrogen , it is called secondary amine . If there is no hydrogen atom attached to nitrogen, it is called tertiary amine. -Now that we know what primary amines are , let us understand what is the type of reaction mentioned in the question. -Gabriel Phthalimide reaction is the conversion of primary alkyl halide groups into their corresponding amine by using potassium Phthalimide as the reagent. -These amines will always be primary in nature -Let us look at the Gabriel Phthalimide general reaction and how it proceeds:
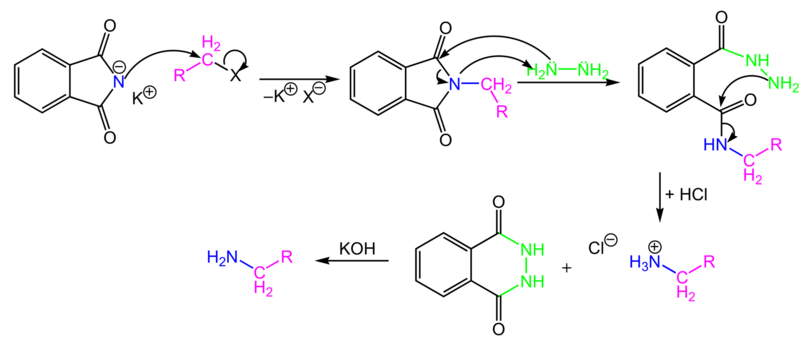
-In the above reaction we can see that primary alkyl halide is converted into primary amine by reacting it with potassium Phthalimide which acts as a nucleophile.
-Since, we know the above reaction can only yield primary aliphatic amine. Aromatic amine cannot be produced in this reaction. Let us write the structure of the given options and eliminate the one which is not aliphatic 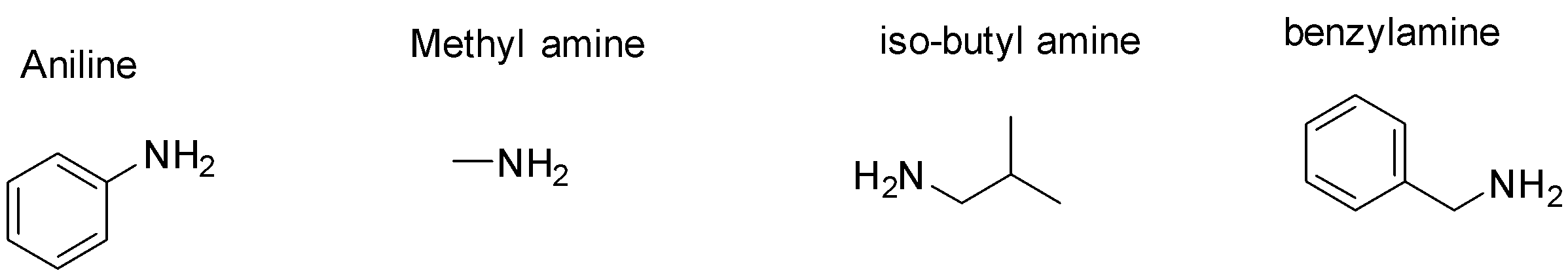 -Out of the given options, Aniline cannot be prepared using Gabriel Phthalimide synthesis since it is not primary amine .
-Out of the given options, Aniline cannot be prepared using Gabriel Phthalimide synthesis since it is not primary amine .
Hence option A is correct.
Note:
Even though benzylamine is aromatic in nature, the amine group in the molecule is not directly attached to the aromatic ring. The amine group in benzylamine is part of the aliphatic side chain and not the aromatic ring and hence it can be prepared via Gabriel Phthalimide synthesis.
