Question
Question: Amine that cannot be prepared by Gabriel phthalimide synthesis is: A. Aniline B. Benzylamine C...
Amine that cannot be prepared by Gabriel phthalimide synthesis is:
A. Aniline
B. Benzylamine
C. Methylamine
D. Iso- butylamine
Solution
Hint: Gabriel phthalimide synthesis is a chemical reaction in which primary alkyl halides are converted into primary amines in the presence of ethanolic potassium hydroxide. In primary alkyl halides or haloalkanes, the halogen atoms such as F, Cl, or Br are bonded to a primary carbon. Using this information, we can solve this question.
Step by Step solution:
In this method, Sodium (Na) or Potassium (K) salt of phthalimide is N-alkylated with a primary (10)alkyl halide to give its corresponding N-alkyl phthalimide.
When acidic hydrolysis takes place, the primary amine is released as the amine salt.
The reaction can also be carried out involving hydrazine. Phthalhydrazide (C6H4(CO)2N2H2) precipitated with the primary amine.
C6H4(CO)NR+N2H4→C6H4(CO)2N2H2+RNH2
In this, phthalimide reacts with ethanolic potassium hydroxide to form the salt. This helps the Nitrogen atoms to get attached with the R group atoms easily.
The sodium or potassium salt of phthalimide on heating with the respective haloalkane followed by an acidic/alkaline hydrolysis gives a primary amine. This transformation can only take place with a primary haloalkane.
If we proceed with the secondary alkyl halides, we won’t be able to get the perfect result. As Gabriel synthesis fails with secondary (20) alkyl halides.
Aromatic primary amines cannot also be formed by this process. A reaction has been shown below for the same -
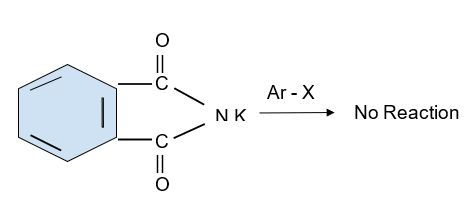
However, except iso-butyl amine all others are primary haloalkanes and can be prepared by Gabriel phthalimide.
Hence, the correct answer is (d) iso-butyl amine.
NOTE – The process of Gabriel phthalimide synthesis only works with primary alkyl halides/haloalkanes. It doesn’t yield useful results in case of secondary haloalkanes. Aromatic amines cannot also be prepared by this method. Keeping this in mind, we can easily figure out which amines can or cannot be formed by this process.
