Question
Question: Aluminium has FCC structure. The edge length of the unit cell is 404pm If the density of the metal i...
Aluminium has FCC structure. The edge length of the unit cell is 404pm If the density of the metal is 2.7gcm−3 , the molar mass (in gmol−1 ) of Al atom is:
A.28.20
B.30.40
C.26.80
D.25.20
Solution
An FCC unit cell contains atoms at all the corners of the crystal lattice and at the center of all the faces of the cube. Basically, the atom present at the face-centered is shared between two adjacent unit cells and only 21 of each atom belongs to an individual cell.
Formula used:
d=NAa3zM
Where d is the density
M is molar mass
a is edge length
NA is Avogadro’s number
Z is the number of formula units in the unit cell.
Complete step by step answer:
In FCC unit cells, the atoms are present in all the corners of the crystal lattice. Moreover, there is an atom present at the center of every face of the cube. The structure is as shown:
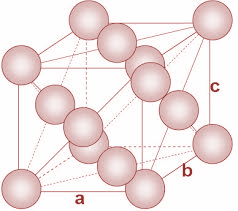
Now, in the given question, we have to calculate the molar mass Al atom. We have been given the density and the edge length of the unit cell.
Now, density is 2.7gcm−3
Edge length i.e. a is 404pm
NA i.e. Avogadro number is 6.023×1023
And z for FCC is 4
So, according to the given formula,
d=NAa3zM
By substituting the values, we get
d=6.023×1023×(404×10−10)34×M
2.7=6.023×1023×(404×10−10)34×M
Therefore, M=26.80gmol−1
Hence, option C is correct.
Note: The smallest replicating portion of a crystal lattice is known as a unit cell. They further exist in many types. The cubic crystal structure for example, consists of three distinct unit cell types i.e. plain cubic, face centered cubic and body centered cubic.
