Question
Question: Aluminium crystallizes in an FCC structure. Atomic radius of the metal is 125pm. What is the length ...
Aluminium crystallizes in an FCC structure. Atomic radius of the metal is 125pm. What is the length of the side of the unit cell of the metal?
Solution
Face centered cubic [FCC] crystal structure has eight spheres at corner of cube and one sphere at center of each face of cube.
Formula used: a=8r
Complete step by step answer: The FCC structure is given as:
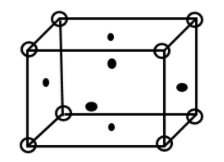
Given that aluminium crystallizes in FCC structure.
Therefore, we first calculate No. of molecules present in FCC structure.
8 molecules present at corner of cube therefore No. of molecule at corner=81×8
6 molecules present on face of cube therefore No. of molecule at faces=21×6 = 3
Total molecule = 3 + 1= 4
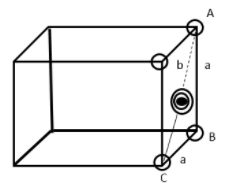
Consider triangle ABC of a side of a face centered cubic unit cell.
The length of edge = a
Hypotenuse AC = b
By Pythagoras theorem
AC2=AB2+BC2
b2=a2+a2
b2=2a2 . . . . . (1)
Since b=4r r=radius of sphere.
Hypotenuse b has one sphere and two half spheres
Putting value of b in equation (1)
(4r)2=2a2
16r2=2a2
a2=216r2=8r2
∴a=8r
In given problem r=125pm.
So length of side of unit cell is
a=8×125
8=4×2
=22
a=22×125
2=1.414
a=2×1.414×125
a = 353.5pm
Therefore, the edge length of face centered cubic structure of aluminium is 353.5 pm.
Note: The relation between edge length of crystal structure and radius of sphere is different in different structures. This is as follows.
Simple cubic a = 2r
Face centered cubic[FCC] a=8r
Body centered cubic[BCC] a=34r
