Question
Question: Although chlorine is an electron withdrawing group yet it is ortho, para directing in nature. Why?...
Although chlorine is an electron withdrawing group yet it is ortho, para directing in nature. Why?
Solution
Chlorine belongs to the halogen family of elements in the periodic table. Its electron withdrawing ability is due to higher electronegativity and has a lone pair of electrons for donation.
Complete step by step answer: Chlorine is an atom in the periodic table with atomic number 17 and electronic configuration[Ne]3s23p5. Chlorine needs only one electron to fill up the octet and has high electron affinity and electronegativity for electrons in bonded or non bonded condition.
Electronegativity is defined as the power of a bonded atom to pull the bonded pair of electrons towards itself. As chlorine is a more electronegative element it attracts the electrons through inductive effect and denoted as – I effect.
Besides this the chlorine atom has seven electrons in its outer valence shell which are available for donation. These electrons take part in resonance with the nearby pi electrons and donate electrons through resonance effect or +R effect. Halogens have the general tendency to show more dominating inducting effect than the resonance effect as they are the most electronegative elements in the periodic table. But in the case of chlorine the case is different. Chlorine has greater resonance effect than inductive effect caused by the electron donating power.
Due to such participation in the conjugation of the lone pair of electrons on the chlorine atom, the chlorine makes the ortho and para position more electron rich than the meta position. This is evident by the resonating structure of chlorobenzene as follows:
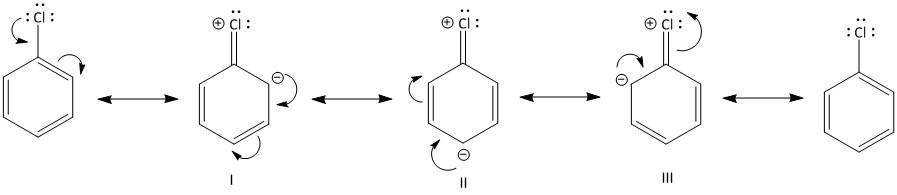
Thus it is clear from the resonating structures I, II and III that the ortho and para are more electron rich positions so chlorine is ortho, para directing in nature.
Note:
Unlike chlorine which under +R effect, groups like NO2 or COOH have –R effect. They pull the electrons of the ring towards itself making the ortho and para position electron deficient than the meta position. Hence these groups are meta directing.
