Question
Question: \[\alpha \] -D-fructofuranose is an aldopentose. A) True B) False...
α -D-fructofuranose is an aldopentose.
A) True
B) False
Solution
We know that D-fructofuranose is a cyclic structure of furanose and it contains one ketonic group and six-carbon atom including the ketonic group. Structures containing the aldehyde group come under aldopentoses.
Complete step by step answer:
Let us see the structure of D-fructose:
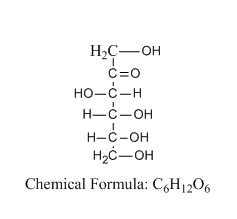
From the structure, we can tell that the structure consists of six carbon atoms and a ketone group at position number 2.
Now let us see the structure of α -D-fructofuranose:
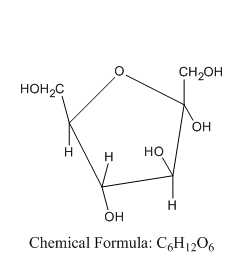
From this, we can say that the α -D-fructofuranose comes under the group of hex ketose and not aldopentose.
Hence the statement is a false statement.
Therefore, we can conclude that the correct answer to this question is option B.
Additional information:
-We know that Fructose is a simple ketone monosaccharide found in plants where it is often bonded to glucose to form the disaccharide sucrose. We also know that fructose is a 6-carbon polyhydroxy ketone and crystalline fructose adopts a cyclic six-membered structure owing to the stability of its hemiketal and internal hydrogen-bonding and this structure is -generally called as D-fructofuranose.
We must know that fructose is highly soluble in water than any other natural form of sugars, as well as other sugar alcohols.
-Hence it is very difficult to crystallize fructose from the aqueous solution.
-Fructose consists of two forms of hemiketal isomers.
-We know that the fructose is found in honey, berries and in maximum number of fruits.
-It is observed that in water solution, fructose exists as a mixture of 70% fructopyranose and about 22% fructofuranose, and also small amounts of three other forms which also includes the acyclic structure.
Note:
In this type of question we have to focus on the structure of the α -D-fructofuranose. Only by seeing the structure, we can find that as it does not contain an aldehyde group it cannot come under the category of aldopentose.
