Question
Question: Allyl isocyanide has: (A) 9σ and 4π – bonds (B) 8σ and 5π – bonds (C) 9σ, 3π and 2 non – bonde...
Allyl isocyanide has:
(A) 9σ and 4π – bonds
(B) 8σ and 5π – bonds
(C) 9σ, 3π and 2 non – bonded electron
(D) 8σ, 3π and 4 non – bonded electron
Solution
Hint: The molecular formula of Allyl isocyanide is C4H4N. The molecular weight of Allyl isocyanide is 67.09g/mol.
Step by step answer:
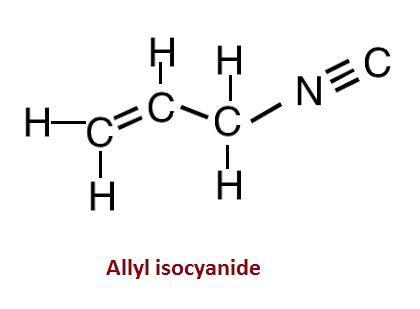
Allyl isocyanide can also be written as CH2=CHCH2N≡C.
We can see that in the given compound there are seven single bonds, one double bond and one triple bond.
!!σ!! bonds are the strongest type of covalent bond. !!σ!! bonds are formed by the head on overlapping atomic orbitals.
!!π!! bonds are covalent bonds in which the two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally.
The double bond has 1 !!σ!! bond and 1 !!π!! bond.
The triple bond has 1 !!σ!! bond and 2 !!π!! bonds.
And there are 2 non bonded electrons.
So in the given compound the number of !!σ!! and !!π!! bonds are 9 and 3 respectively with 2 non bonded electrons.
Therefore, Option C is the correct answer.
Additional Information: !!σ!! bonds results from the head on overlap of the two orbitals of the two atoms, which has more area of overlap than the !!π!! bonds which result from the parallel overlap of orbitals. That is why !!σ!! bonds are stronger than !!π!! bonds.
Note: The formula to calculate !!σ!! bonds is: X+Y-1
Where,
X = Number of Carbon atoms
Y = Number of Hydrogen atoms
The formula to calculate !!π!! bonds is: \left[ \left\\{ \dfrac{\left( \text{2X-Y} \right)}{\text{2}} \right\\}\text{+1} \right]
Where,
X = Number of Carbon atoms
Y = Number of Hydrogen atoms
