Question
Question: If both the containers are maintained at constant temperature (R = 0.0821 L-atm/K-mol) Container-I:...
If both the containers are maintained at constant temperature (R = 0.0821 L-atm/K-mol)
Container-I: 300 K, 2 mol H₂, 16.42 lit. Container-II: 400 K, 1 mol H₂, 8.21 lit.
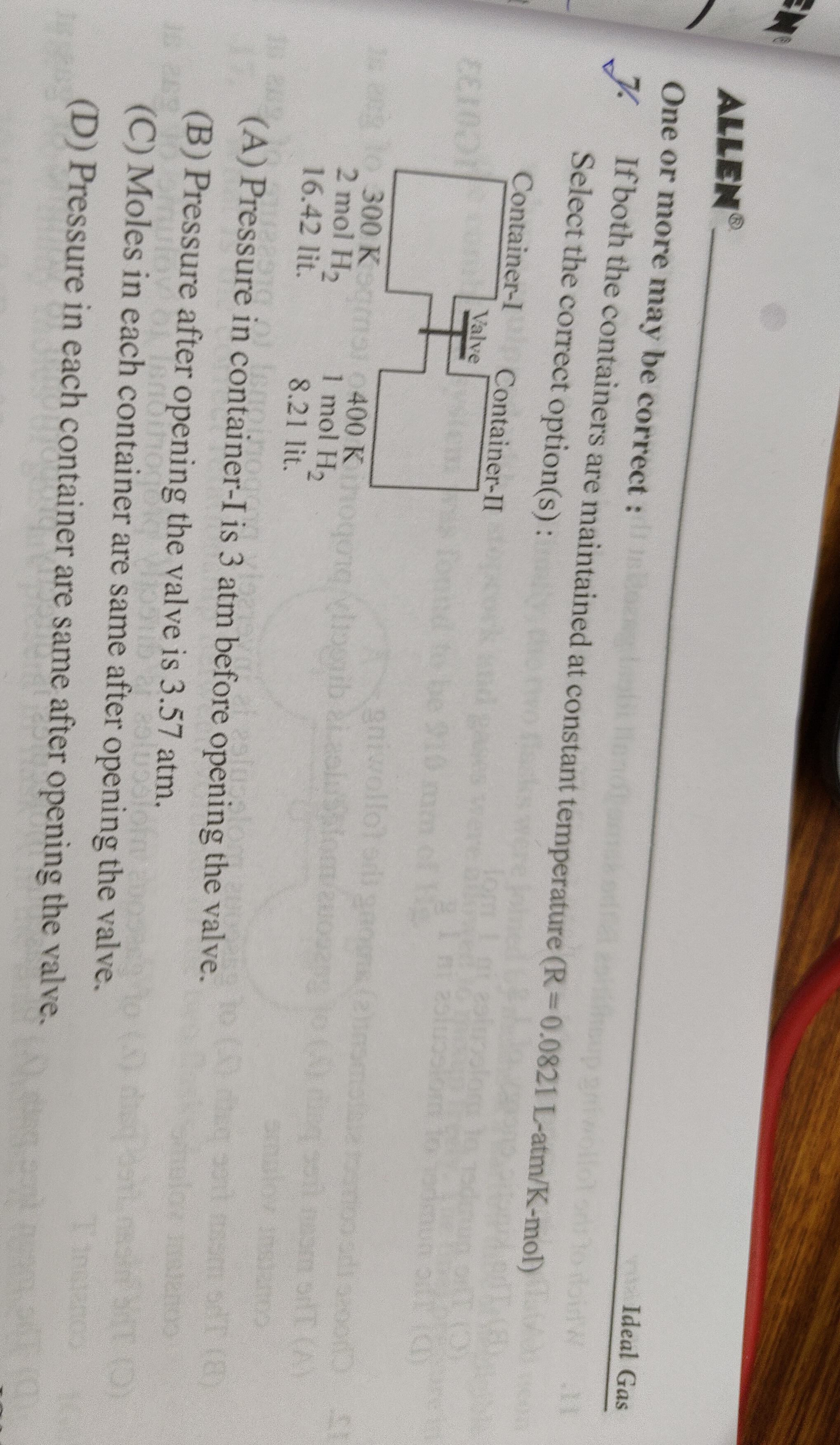
Pressure in container-I is 3 atm before opening the valve.
Pressure after opening the valve is 3.57 atm.
Moles in each container are same after opening the valve.
Pressure in each container are same after opening the valve.
A, B, D
Solution
Calculate initial pressures using the ideal gas law: P1=V1n1RT1=16.422×0.0821×300=3 atm. P2=V2n2RT2=8.211×0.0821×400=4 atm. After opening the valve, ntotal=2+1=3 mol and Vtotal=16.42+8.21=24.63 L. The final temperature is the weighted average of initial temperatures: Tf=P1+P2P1T1+P2T2=3+43×300+4×400=7900+1600=72500 K. The final pressure is Pf=VtotalntotalRTf=24.633×0.0821×(2500/7)≈3.57 atm. If the final temperature is uniform, the pressure is uniform throughout the combined volume.
