Question
Question: Which of the following options correctly mention the configuration which is most stable among Q and ...
Which of the following options correctly mention the configuration which is most stable among Q and R.
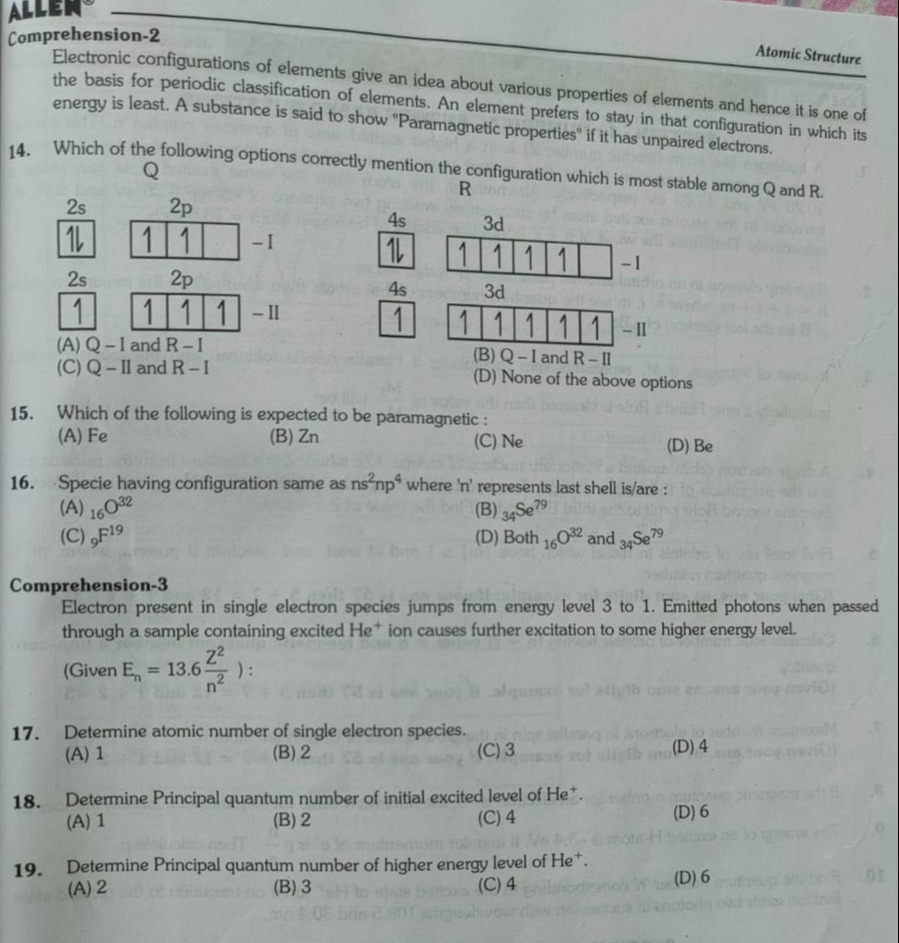
A
Q – I and R – I
B
Q – I and R – II
C
Q – II and R – I
D
None of the above options
Answer
(A) Q – I and R – I
Explanation
Solution
-
For Q:
- Q-I: 2s² 2p³ (5 valence electrons). This configuration has a fully filled 2s subshell and a half-filled 2p subshell. Half-filled and fully-filled subshells are exceptionally stable due to symmetry and exchange energy.
- Q-II: 2s¹ 2p⁴ (5 valence electrons). This configuration has a partially filled 2s subshell and a partially filled 2p subshell (neither half nor fully filled).
- Therefore, Q-I (2s² 2p³) is more stable than Q-II (2s¹ 2p⁴).
-
For R:
- R-I: 4s¹ 3d⁵ (6 valence electrons). This is the actual ground state electronic configuration of Chromium (Cr, Z=24). It achieves extra stability by having a half-filled 3d subshell and a half-filled 4s subshell.
- R-II: 4s² 3d⁴ (6 valence electrons). This would be the configuration predicted by the Aufbau principle, but it is less stable than R-I due to the absence of half-filled stability.
- Therefore, R-I (4s¹ 3d⁵) is more stable than R-II (4s² 3d⁴).
Conclusion: Q-I and R-I are the most stable configurations.
