Question
Question: Select the option representing correct order of basicity....
Select the option representing correct order of basicity.
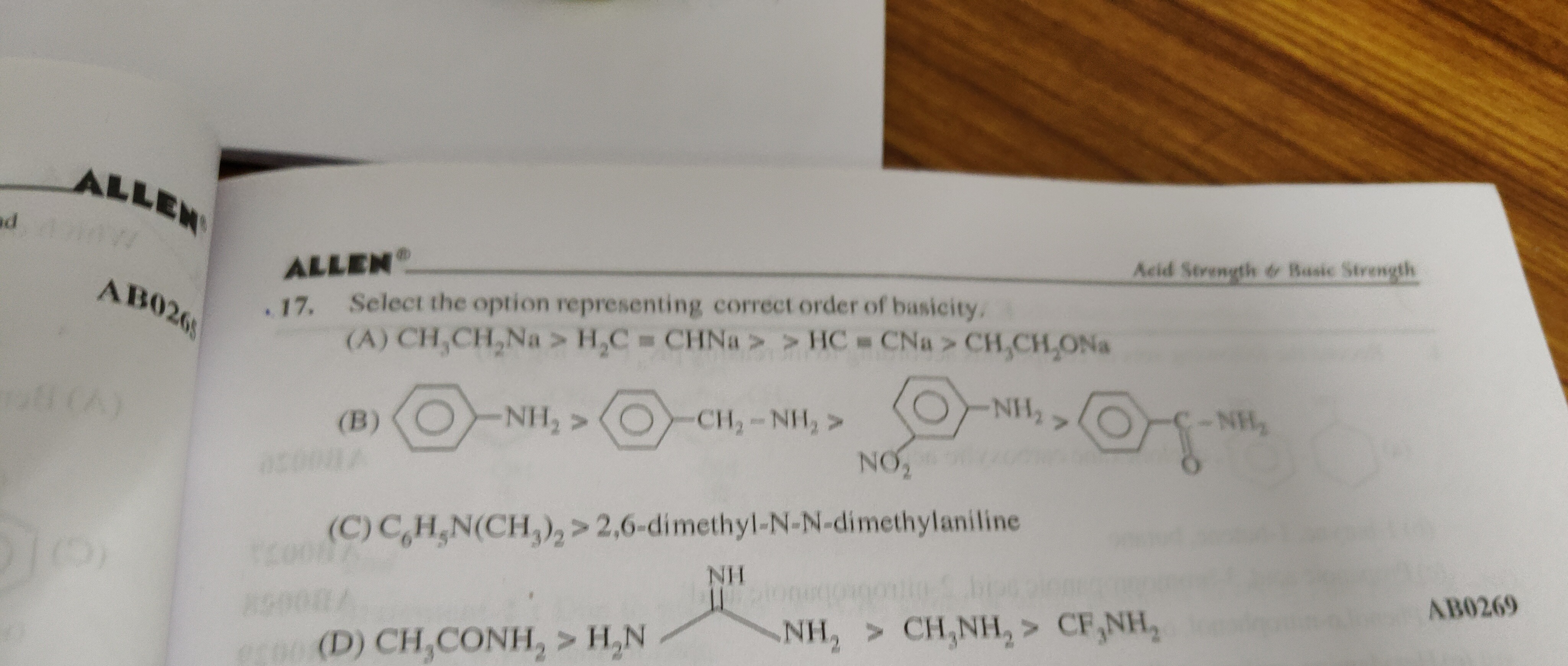
CH3CH2Na>H2C=CHNa>HC=CNa>CH3CH2ONa
Figure representing a set of compounds with an order of basicity.
C6H5N(CH3)2>2,6-dimethyl-N-N-dimethylaniline
CH3CONH2>H2N NH2>CH3NH2>CF3NH2
The correct options are (A) and (C).
Solution
The basicity of a compound is determined by the availability of the lone pair of electrons on the basic atom. Higher electron density on the basic atom or greater stability of the conjugate acid leads to higher basicity.
Option (A): The species are sodium salts, which dissociate to form Na+ and an anion (R− or RO−). The basicity of these salts is determined by the basicity of the corresponding anions. Basicity of an anion is inversely proportional to the acidity of its conjugate acid. The conjugate acids are:
- CH3CH2Na: conjugate acid is CH3CH3 (ethane, sp3 C-H)
- H2C=CHNa: conjugate acid is H2C=CH2 (ethene, sp2 C-H)
- HC=CNa: conjugate acid is HC≡CH (ethyne, sp C-H)
- CH3CH2ONa: conjugate acid is CH3CH2OH (ethanol, O-H)
The acidity of C-H bonds increases with the s-character of the carbon atom: sp3<sp2<sp. The O-H bond in alcohols is much more acidic than C-H bonds. Approximate pKa values: Ethane (~50), Ethene (~44), Ethyne (~25), Ethanol (~16). Order of acidity: Ethanol > Ethyne > Ethene > Ethane. Therefore, the order of basicity of their conjugate bases (anions) is: Ethoxide anion < Acetylide anion < Vinyl anion < Ethyl anion. This translates to the basicity order of the sodium salts as: CH3CH2ONa<HC=CNa<H2C=CHNa<CH3CH2Na. Thus, the order given in option (A) is correct: CH3CH2Na>H2C=CHNa>HC=CNa>CH3CH2ONa.
Option (C): The compounds are N,N-dimethylaniline (C6H5N(CH3)2) and 2,6-dimethyl-N,N-dimethylaniline. In N,N-dimethylaniline, the lone pair on nitrogen is delocalized into the phenyl ring through resonance, reducing its basicity. The methyl groups are electron-donating by induction. In 2,6-dimethyl-N,N-dimethylaniline, the two methyl groups at the ortho positions cause significant steric hindrance, which impedes protonation and also reduces the extent of resonance. This steric effect makes it a weaker base than N,N-dimethylaniline. Therefore, the order of basicity is C6H5N(CH3)2>2,6-dimethyl-N-N-dimethylaniline. Option (C) is correct.
