Question
Question: Column I & Column II contain data on Schrodinger Wave-Mechanical model, where symbols have their usu...
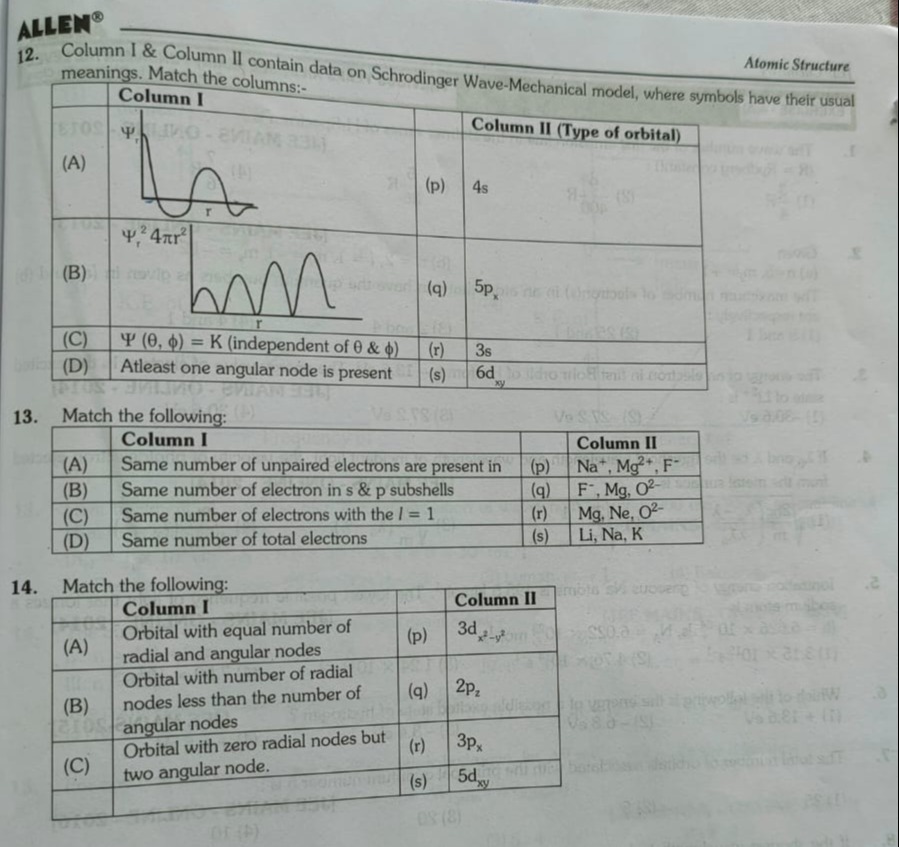
Column I & Column II contain data on Schrodinger Wave-Mechanical model, where symbols have their usual meanings. Match the columns:-
| Column I | Column II (Type of orbital) | ||
|---|---|---|---|
| (A) | (p) | 4s | |
| ψr24πr2 | |||
| (B) | (q) | 5px | |
| (C) | Ψ(θ,ϕ)=K (independent of θ & ϕ) | (r) | 3s |
| (D) | Atleast one angular node is present | (s) | 6dxy |
A
A - r
B
B - q
C
C - p
D
D - s
Answer
A - r, B - q, C - p, D - s
Explanation
Solution
Question 12:
- (A) ψr vs r graph: The graph shows 2 radial nodes (where ψr crosses the r-axis). For a 3s orbital (n=3, l=0), the number of radial nodes is n−l−1=3−0−1=2.
- (B) ψr24πr2 vs r graph: The graph shows 3 radial nodes (where the probability density is zero) and 4 peaks. For 5p orbital (n=5, l=1), radial nodes = 5−1−1=3, and number of peaks = n−l=5−1=4.
- (C) Ψ(θ,ϕ)=K: This implies the angular part of the wave function is constant, meaning it is spherically symmetric. This is characteristic of s-orbitals (l=0). The 4s orbital is the only remaining s-orbital option.
- (D) At least one angular node: An angular node is present when l≥1. The 6dxy orbital has l=2, hence it has two angular nodes (which is at least one).
