Question
Question: All the hydrocarbons shown are weak acids. One, however, is far more acidic than the others. Which o...
All the hydrocarbons shown are weak acids. One, however, is far more acidic than the others. Which one is the strongest acid?
(A) 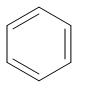
(B) 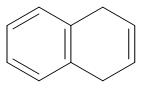
(C) 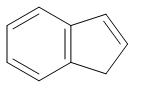
(D) 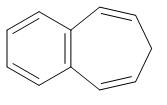
Solution
In the above question, all hydrocarbons are weak acids. We have to choose the compound which is strongest amongst them. This can be analysed by the number of free hydrogen ions present in each of the compounds. More the easier to free hydrogen from the compound, the more acidic the character.
Complete Step by step solution:
Let us first look at what is an acid.
An acid can be defined as a molecule or ion that is capable of donating a proton or H + ion or, alternatively, the one which is capable of forming a covalent bond with an electron pair.
Now, let us see in which of the compounds the removal of hydrogen ions is readily feasible as compared to others.
We can clearly see that benzene is the only compound from which removal of hydrogen ions is comparatively easier. In the rest of the compound, removal of hydrogen ions is not that feasible because of the presence of bulky groups.
Hence, we can say that benzene is the strongest acid among the given hydrocarbons.
Hence, option A is the correct option.
Note:
Benzene is used in the preparation of phenol. It is also used to prepare aniline which are used in dyes and in dodecylbenzene which are used for the detergents. Earlier, it was used in degreasing of metal.
Benzene storage is really a matter of consideration as acute exposure to benzene may produce toxic effects on the central nervous system.
