Question
Question: All of the following reactions are non-elementary reactions. Which of the following is the best fi...
All of the following reactions are non-elementary reactions.
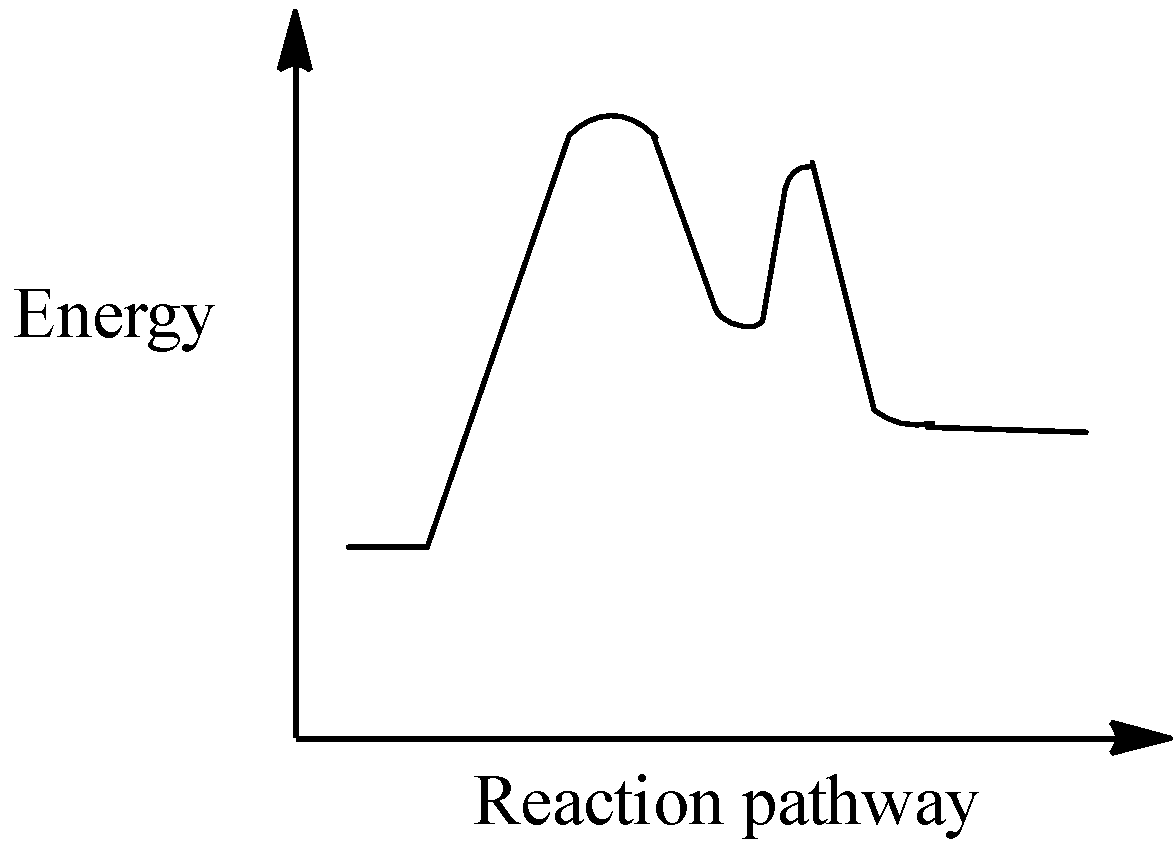
Which of the following is the best fit reaction for the following energy diagram?
A) The reaction between aluminium and copper (II) sulphate solution with a ΔH=−12.37kJ/mol
B) The reaction that forms hydrogen and nitrogen from ammonia all in the gas state with a ΔH=30.50kJ/mol
C) The dissolution of sodium hydroxide in water with aΔH=−44.51kJ/mol
D) The ionisation of hydrochloric acid to make a solution with aΔH=−74.84kJ/mol
Solution
The answer to this question is based on the concept of potential energy diagram and therefore depends on the comparison energy of the reactants and product which assigns either positive or negative value to the enthalpy of reaction.
Complete Solution :
The concept of the potential energy diagram is familiar to us from the chapters in the physical chemistry section and also we have studied about the types of reactions that are exothermic or endothermic reactions.
- Now, let us focus on the potential energy diagram and about the information that can be obtained by this diagram which will help us to obtain the correct answer.
- Basically a potential energy diagram is the graph from which we can illustrate the mechanism for a reaction which is plotted as energy versus the reaction pathway or reaction coordinate.
- Non elementary reaction is the one in which there is no direct correspondence between the stoichiometry and the rate of reaction.
Thus, in the above given graph depicts that the energy of the reactant molecules is lesser than that of the products and hence the reaction is endothermic in nature.
This means that the enthalpy of the formation of products will be higher than the enthalpy of the reactants in the system. Thus the value will be positive, that is ΔH is positive.
- The reaction that forms hydrogen and nitrogen from ammonia all in the gas state with a ΔH=30.50kJ/mol
So, the correct answer is “Option B”.
Note: Note that the difference between elementary and non elementary reactions is as follows, elementary reactions are those reactions that are well defined reactions which result from the single collision between two molecules or ions and non elementary reactions consist of a series of the elementary reactions.
