Question
Question: All of the following have a tetrahedral shape except : a.) \(S{O_4}^{2 - }\) b.) \(Xe{F_4}\) ...
All of the following have a tetrahedral shape except :
a.) SO42−
b.) XeF4
c.) ClO4−
d.) XeO4
Solution
Hint: Try to draw the Lewis structures for all these compounds given in the question and then using the Valence Shell theory, try to figure out the shape of the molecules. The VSEPR theory assumes that all electron pairs, both bonding pairs and lone pairs, repel each other, particularly if they are close and that the molecular shape is such as to minimize these repulsions.
Complete answer:
The theory of molecular shape which is often known as the valence-shell electron-pair repulsion (VSEPR) theory grew out of Lewis's theory, and, like that approach to bonding, VSEPR focuses on the role of electron pairs. It imparts the bonding influences to the electron pairs that lie between atoms and acknowledges the existence of lone pairs of electrons that do not directly participate in the bonding.
Sulphate ion (SO42−) can be treated as having the equivalent of four pairs (two ordinary pairs and two superpairs) around the sulphur atom in a tetrahedral arrangement. Since all the four pairs are bonding clearly in the diagram on the next page, the ion is predicted to be a regular tetrahedron.
Xenon undergoes the sp3 hybridization in the fourth excited state when in the formation of XeO4. In the fourth excited state, the xenon atom has 8 unpaired electrons. Out of all one “s” and three “p” orbitals undergo sp3 hybridization. Because of the fact that it has no lone pair of electrons, the shape of XeO4 is tetrahedral with the bond angle of 109 degrees.
In the VSEPR structure of perchlorate ion ClO4−. There are 32 valence electrons available for constructing the Lewis structure for ClO4−. Putting chlorine at the centre as it is the least electronegative. As chlorine can hold more than 8 valence electrons it makes three double bonds with the 3 oxygen atoms. Around the chlorine atom in ClO4−, there are four σ(sigma) bonds with zero lone pairs. Therefore the shape of the anion around the chlorine atom is tetrahedral and so is the shape of the perchlorate ion.
The VSEPR (Valence shell electron pair repulsion theory) structure of XeF4 is square planar due to presence of lone pair whereas the rest of the given have tetrahedral shape as shown below –
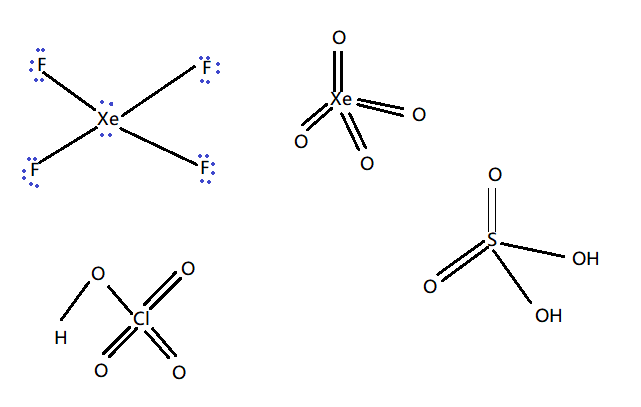
XeF4, Clearly consists of two lone pairs of electrons. Now according to the VSEPR theory, the bond pair electrons and lone pair electrons will experience a repulsive force between them. Because of these repulsive forces, they acquire a stable state. The lone pairs of Xenon are located in the perpendicular plane in an octahedral pattern.
Therefore, XeF4 molecular geometry is square planar and not tetrahedral.The bond angles are 90 or 180°. The lone pairs lie on the opposite sides of the molecule basically at 180° from each other.
Note: While predicting the shapes of the molecules using VSEPR theory -
The least electronegative atom in the molecule must be selected as the central atom .
The total number of electrons belonging to the outermost shell of the central atom must be counted.
The total number of electrons belonging to other atoms and used in bonds with the central atom must be counted.
These two values must be summed up in order to obtain the valence shell electron pair number or also known as the VSEP number.
