Question
Question: Aldol condensation product of acetone on dehydration gives: (a)- But-2-enal (b)- 2–Methyl-pent–3...
Aldol condensation product of acetone on dehydration gives:
(a)- But-2-enal
(b)- 2–Methyl-pent–3–en–4–one
(c)- 4–Hydroxy–4–methyl pentan–2 –one
(d)- 4–Methyl–pent–3–en–2–one
Solution
Aldol condensation is a process of addition of molecules in carbonyl compounds, i.e., aldehydes and ketones, this process is only possible if the compound has an alpha-hydrogen atom. On dehydration, there will be the removal of the water molecule, forming a double bond in the compound.
Complete answer:
Acetone is a compound having a carbonyl group, i.e., (>C=O), or more specifically saying it is a ketone having three carbon atoms and the functional group is at the second carbon atom. The structure is given below:
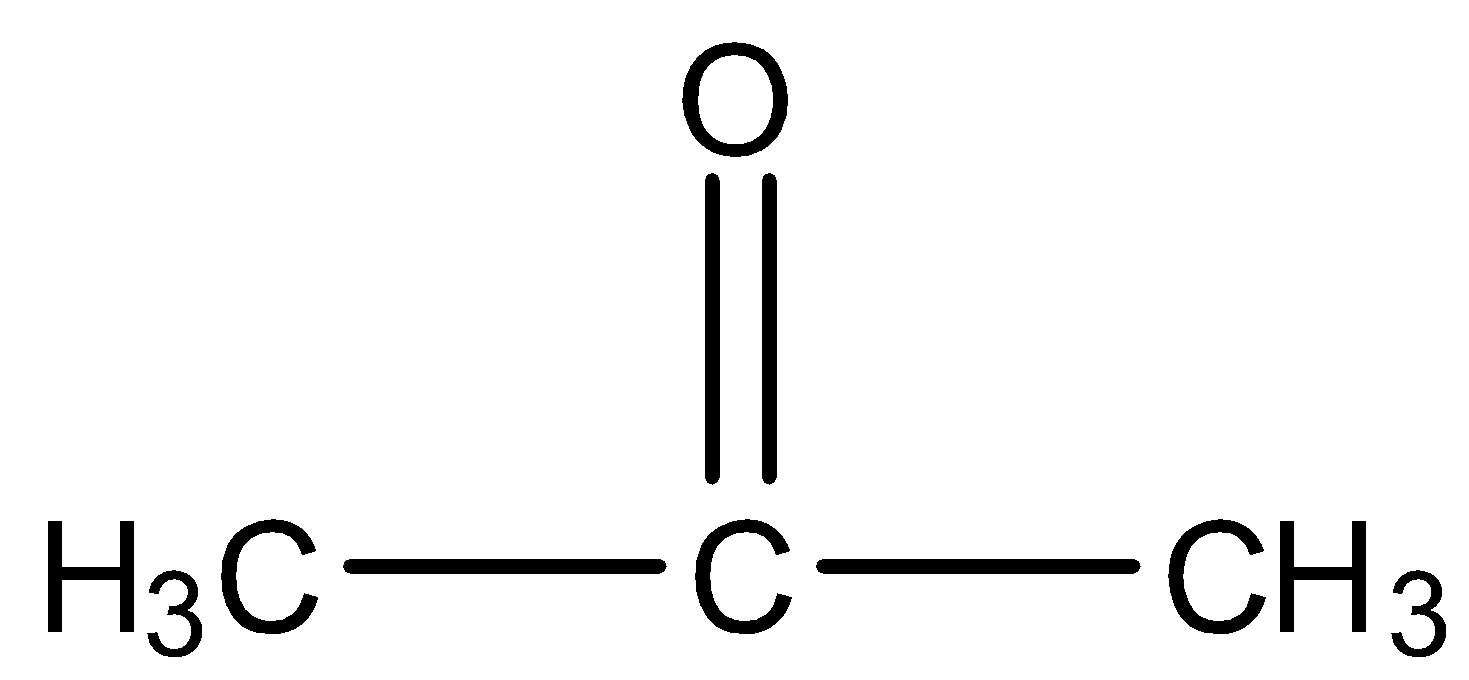
Aldol condensation is a process of the addition of molecules in carbonyl compounds, i.e., aldehydes and ketones, this process is only possible if the compound has an alpha-hydrogen atom. So, when two moles of acetone undergo aldol condensation in the presence of alkali then the alpha-hydrogen of one molecule of acetone will attack the oxygen atom of the other molecule and the rest part will get attach to the carbon atom having the oxygen atom. The reaction is given below:
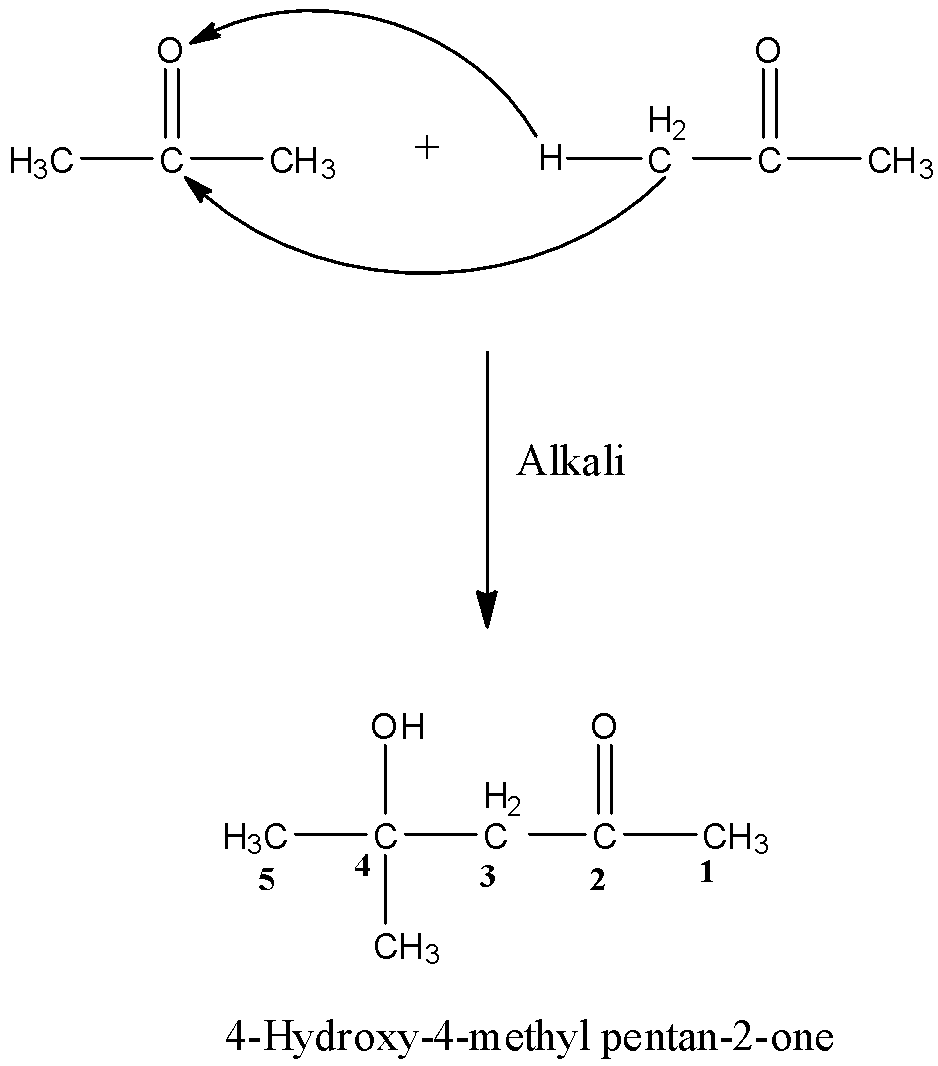
The given product is 4–Hydroxy–4–methyl pentan–2 –one, and when this is dehydrated then there is the removal of a water molecule. The OH from the 4th carbon atom and H from the 3rd carbon atom and will form a double bond. The reaction is given below:
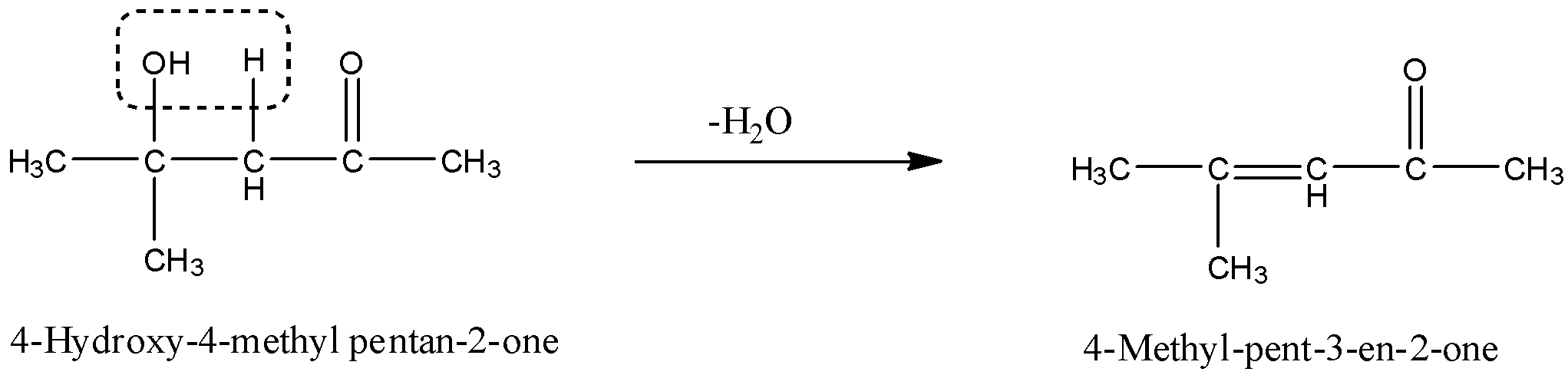
So, the formed product is 4–Methyl–pent–3–en–2–one.
Therefore, the correct answer is an option (d)- 4–Methyl–pent–3–en–2–one.
Note:
The aldol condensation is only possible in the alkaline medium. If two same molecules are there in the reaction, then it is called self-aldol condensation, and if two different molecules are there in the reaction then it is called cross-aldol condensation.
