Question
Question: 9. $\xrightarrow[\Delta]{K_2Cr_2O_7} (P)$; Product $(P)$ is:...
K2Cr2O7Δ(P); Product (P) is:
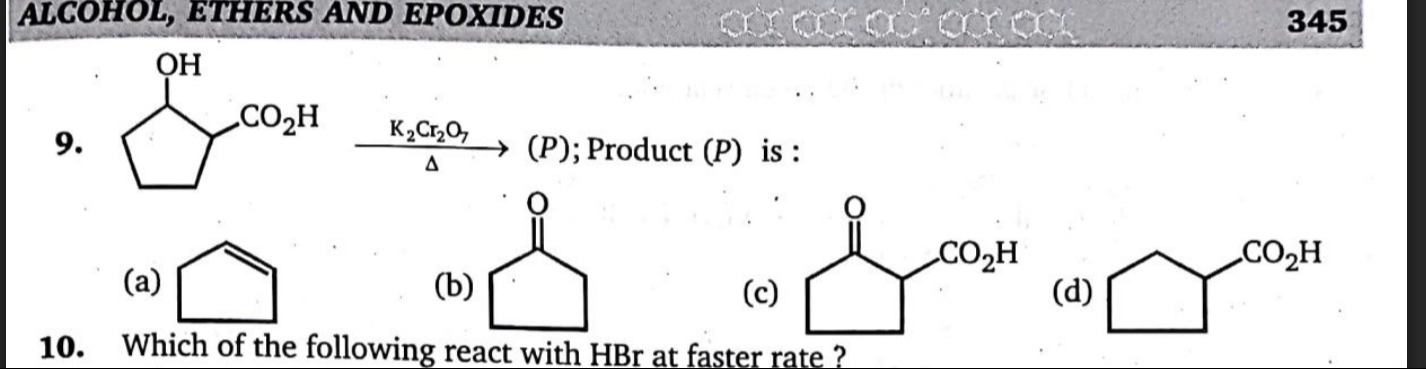
Cyclopentene
Cyclopentanone
2-oxocyclopentane-1-carboxylic acid
Cyclopentane-1-carboxylic acid
2-oxocyclopentane-1-carboxylic acid
Solution
The given reaction involves the oxidation of 2-hydroxycyclopentane-1-carboxylic acid with potassium dichromate (K2Cr2O7) and heat (Δ).
-
Identify the functional groups in the reactant:
The reactant is a cyclic compound containing two functional groups:- A secondary alcohol (-OH group attached to a carbon that is bonded to two other carbons).
- A carboxylic acid (-COOH group).
-
Understand the role of the reagent (K2Cr2O7/Δ):
Potassium dichromate in acidic medium (implied, as it's a common strong oxidizing agent) is a powerful oxidizing agent.- It oxidizes primary alcohols to carboxylic acids.
- It oxidizes secondary alcohols to ketones.
- It generally does not oxidize tertiary alcohols.
- Carboxylic acids are already in a high oxidation state and are resistant to further oxidation under these conditions.
-
Predict the transformation of each functional group:
- The secondary alcohol group (-CH(OH)-) will be oxidized to a ketone group (-C(=O)-).
- The carboxylic acid group (-COOH) will remain unchanged.
-
Draw the structure of the product (P):
Starting with 2-hydroxycyclopentane-1-carboxylic acid:OH | CH / \ CH2--CH -- COOH | | CH2--CH2
Upon oxidation, the -CH(OH)- group becomes -C(=O)-:
```
O
||
C
/ \
CH2--CH -- COOH
| |
CH2--CH2
```
This product is 2-oxocyclopentane-1-carboxylic acid (or cyclopentanone-2-carboxylic acid).
- Compare with the given options:
- (a) Cyclopentene: This is an alkene, formed by dehydration, not oxidation.
- (b) Cyclopentanone: This is a ketone, but the carboxylic acid group is missing.
- (c) 2-oxocyclopentane-1-carboxylic acid: This structure matches our predicted product, where the secondary alcohol is oxidized to a ketone and the carboxylic acid group remains intact.
- (d) Cyclopentane-1-carboxylic acid: This compound lacks the oxygen at the 2-position, indicating reduction or removal of the alcohol, not oxidation.
Therefore, product (P) is 2-oxocyclopentane-1-carboxylic acid, which corresponds to option (c).
