Question
Question: \[AlC{l_3}\] behave as an Acid in accordance with: A) Arrhenius concept B) Bronsted -Lowery con...
AlCl3 behave as an Acid in accordance with:
A) Arrhenius concept
B) Bronsted -Lowery concept
C) Lewis concept
D) All of these
Solution
Generally Acids are those substances which Turns blue litmus into red & are Sour in taste. They also show electrical conductance and give neutralization reaction when reacts with base.
Complete answer:
AlCl3 is electron deficient. It has three electrons in its valence shell. So when it forms a covalent compound with chlorine it forms three single bonds with chlorine. Three electrons of aluminium and three electrons of chlorine constitute 6 electrons in outermost orbit. So, It doesn't form an octet ( eight electrons in outermost orbit) by doing so, thus it can take two more electrons by forming a coordinate bond to complete an octet and thus it behaves as Lewis acid.
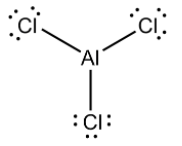
Lewis acid is defined as a compound which can take electrons from a donor compound. These compounds have a tendency to accept lone pairs. This is according to the Lewis concept.
Hence, option (C) is correct.
Note: Some other concepts related to acid are Arrhenius concept & Bronsted – Lowry concept.
According to Arrhenius, Acids are compounds which release H+ ion in aqueous solution.
HCl + H2O→H(aq)+(aq.) + Cl(aq)−
While Bronsted – Lowry concepts states that the compounds which have a tendency to release protons in any solvent are acids.
HCl(acid) + H2O (solvent)→H3O++ Cl−
