Question
Question: \(AlC{{l}_{3}}\)achieves stability by forming a dimer. In the trivalent state, the compound is hydro...
AlCl3achieves stability by forming a dimer. In the trivalent state, the compound is hydrolysed in water. AlCl3 in acidified aqueous solutions forms:
(A)- Al(OH)3+HCl
(B)- [Al(H2O)6]3++3Cl−
(C)- AlCl3.2H2O
(D)- Al2O3+HCl
Solution
The coordination number of Al metal is 6. There are two different types of bonds formed of which one gets ionized in aqueous solution.
Complete step by step answer:
-Aluminium trichloride or aluminium chloride is a chemical compound with aluminium and chloride atoms in a ratio 1:3. Another form which contains six waters of hydration is also available.
-The trifluorides of Al, Ga, In and Tl are ionic while the chlorides, bromides and iodides are largely covalent in the anhydrous state. The covalent nature decreases down the group. Trihalides fume in air and undergo hydrolysis.
-The tendency of accepting an electron pair is observed in this case. The tendency decreases as the size of the cation increases.
-They form complex halides like [MX6]3− where a metal atom has the coordination number equal to 6 by the use of d- orbitals. There is no back bonding due to an increase in the size of the element.
They use vacant p – orbitals to form coordinate bonds to complete their octets thus forming dimers.
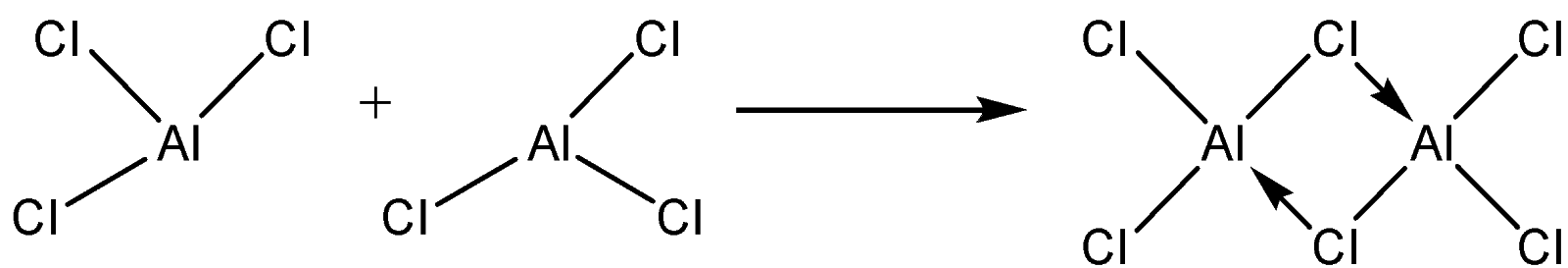
-Dimers formed exist in a vapour state and in non-polar solvents.
-Dimers disappear when the halides are dissolved in water. This is due to high hydration energy.
AlCl3+H2O→[Al(H2O)6]3++3Cl3−
So, the correct answer is “Option B”.
Note: The halides have a planar sp2hybrid structure. The AlCl3 behaves as a Lewis acid. It is borderline for being ionic or covalent. AlCl3 when solid is usually ionic and when in the vapour is covalent. Aluminium chloride in its +3 oxidation state makes six electrons ( 3 electrons from Al and 3 electrons from Cl) to get the most favourable 8 electron octet and thus forms dimmers by extra sharing of electrons.
