Question
Question: \(Al{F_3}\)is soluble in \(HF\)only in presence of \(KF\). It is due to the formation of: A. \(Al{...
AlF3is soluble in HFonly in presence of KF. It is due to the formation of:
A. AlH3
B. K[AlF3H]
C. K3[AlF3H3]
D. K3[AlF6]
Solution
AlF3 is soluble only in presence of HF only in presence of KF. It is due to formation of a complex K3[AlF6],
Complete step by step answer: Aluminium form fluoride complexes more easily than in the case of silicon. So, AlF3 easily dissolves in a mixture of (HF+KF).
AlF3 is soluble in HF due to the formation of K3[AlF6]
AlF3+3KFHFK3[AlF6] ……. (i)
AlF3 is insoluble in anhydrous HF because the F−ions are not available in intermolecular hydrogen bonded HF but it becomes soluble in the presence of KF due to the formation of soluble complex, K3[AlF6].
We can understand it from the equation (i).
Therefore, the correct option is (D) K3[AlF6].
Additional Information:
We can say that –
-Anhydrous HF is a covalent compound and is strongly hydrogen bonded. Therefore, it doesn’t give F−ions and hence AlF3 doesn’t dissolve in HF. NaF is an ionic compound. It contains F−ions which combine with electron deficient AlF3 to form the soluble complex,
3NaF+AlF3→Na3[AlF6] (sodium hexafluoroaluminate (iii))
-Due to Boron’s small size and higher electronegativity it has greater tendency to form complexes than Aluminium. Hence, precipitation of AlF3 takes place when BF3 is passed through Na3[AlF6]
Na3[AlF6]+3BF3→3Na[BF4]+AlF3 {sodium tetrafluoroborate (iii)}
Note: K3[AlF6] (Potassium hexafluoroaluminate) has structure.
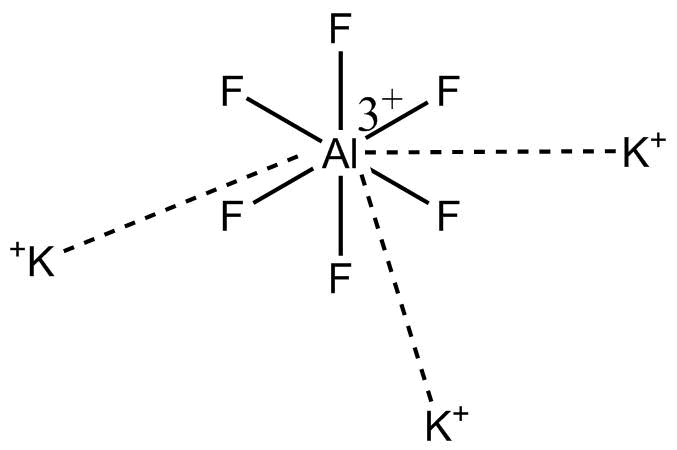
This compound is a compound which can be canonicalized.
