Question
Question: Air pollutant that occurs in sunlight is: A. Oxidizing smog B. Acid rain C. Reducing agent D...
Air pollutant that occurs in sunlight is:
A. Oxidizing smog
B. Acid rain
C. Reducing agent
D. Fog
Solution
. Few chemicals do not present in the atmosphere in excess amounts but they are released by the human action into the air and they pollute the air. The chemicals which do air pollution are called air pollutants. The air pollutants react with other chemicals in presence of sunlight and produce new pollutants.
Complete step by step answer:
- We have to find the air pollutant that occurs in sunlight among the given options.
- Coming to the given options, option A, Oxidizing smog. The chemical, PAN (peroxyacetyl nitrate). PAN is going to be released by the reaction of hydrocarbons, oxygen and nitrogen dioxide in presence of the sunlight.
- The chemical reaction of hydrocarbons, oxygen and nitrogen dioxide in presence of the sunlight is as follows.
Hydro carbons+O2+NO2Sun lightPANCH3COOONO2
- We can see clearly that the above is called oxidation because oxygen is reacting with hydrocarbons and forming respective oxygen derivatives.
- The above oxidation reaction is occurring in sunlight and producing an air pollutant PAN then it is called oxidizing smog.
- Coming to option B, Acid rain. For acid rains there is no need for sunlight because oxides of sulphur and oxides of nitrogen react with water and cause acid rain. So, option B is wrong.
- Coming to option C, maximum air pollutants undergo oxidation reaction in presence of sunlight then option C is wrong.
-Coming to the option D, Fog. It is an aerosol that contains tiny water droplets in the air. There is no relation between sunlight and fog. So, option D is also wrong.
- Therefore Air pollutant that occurs in sunlight is Oxidizing smog.
So, the correct answer is “Option A”.
Note: The structure of the PAN is as follows.
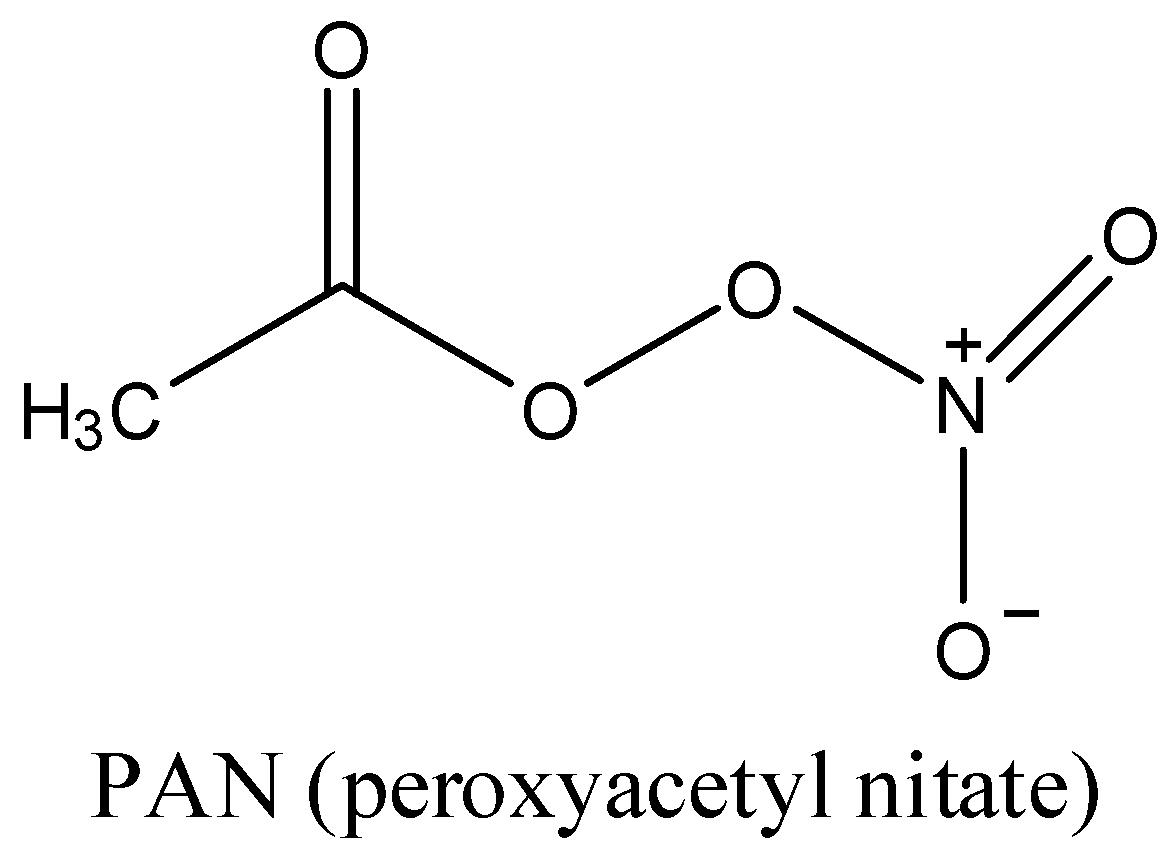
- PAN contains a peroxide bond in its structure. PAN is toxic and causes irritation to the eyes. It readily dissolves in water. PAN is a best example for secondary air pollutants.
