Question
Question: Air is trapped in the space (labeled A) above the mercury level in the tube of a barometer, which me...
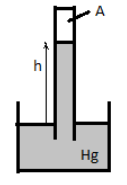
Air is trapped in the space (labeled A) above the mercury level in the tube of a barometer, which measures 745mm Hg. If the atmospheric pressure is 760mm Hg, then the pressure of the air trapped is
A. 15mm Hg
B. 760mm Hg
C. Between 745 and 760mm Hg
D. 745mm Hg
Solution
Use the concept of pressure measurement by a barometer using the height of the mercury level in the tube of the barometer. When one tries to measure the pressure of a liquid in a beaker, the pressure exerted by the atmosphere on the liquid is equal to the sum of the pressure exerted by the mercury on the liquid and the air trapped in the tube of the barometer.
Complete step by step solution:
The mercury level in the barometer shows that pressure 745mm Hg. Air is trapped above the mercury level in the tube of the barometer and the atmospheric pressure is 760mm Hg.
Patm=760mm Hg
P=745mm Hg
The pressure exerted Patm by the atmosphere on the mercury in the beaker and the pressure Pair exerted by the air trapped in the tube of the barometer and pressure Pexerted by the mercury in the tube A must be equal.
Patm=Pair+P
Rearrange the above equation for the pressure Pair of the air trapped in the tube of the barometer.
Pair=Patm−P
Substitute 760mm Hg for Patmand 745mm Hg for P in the above equation.
Pair=(760mm Hg)−(745mm Hg)
⇒Pair=15mm Hg
Therefore, the pressure of the air trapped in the tube of the barometer is 15mm Hg.
Hence, the correct option is A.
Additional information: The barometer measures the air pressure by balancing the weight of the air particles on the tube of the barometer and the weight of the mercury in the barometer.
If the weight of the air particles above the tube of barometer is more than the weight of the mercury then the level of the mercury drops and if the weight of the air particles above the tube of barometer is less than the weight of the mercury then the level of the mercury rises in the tube.
Note: The two pressures i.e. the atmospheric pressure and the sum of the pressure due to mercury in the barometer tube and pressure due to the air trapped in the tube must be equal otherwise the height of the mercury level in the barometer tube would not be steady.
