Question
Question: For reaction: A → 3B, takes place as per first order kinetics, the time in which the concentration o...
For reaction: A → 3B, takes place as per first order kinetics, the time in which the concentration of reactant and product, become equal is (nearest integer)
(Given: k = 4.6×10−2 min−1, log 3 = 0.48, log 2 = 0.3)
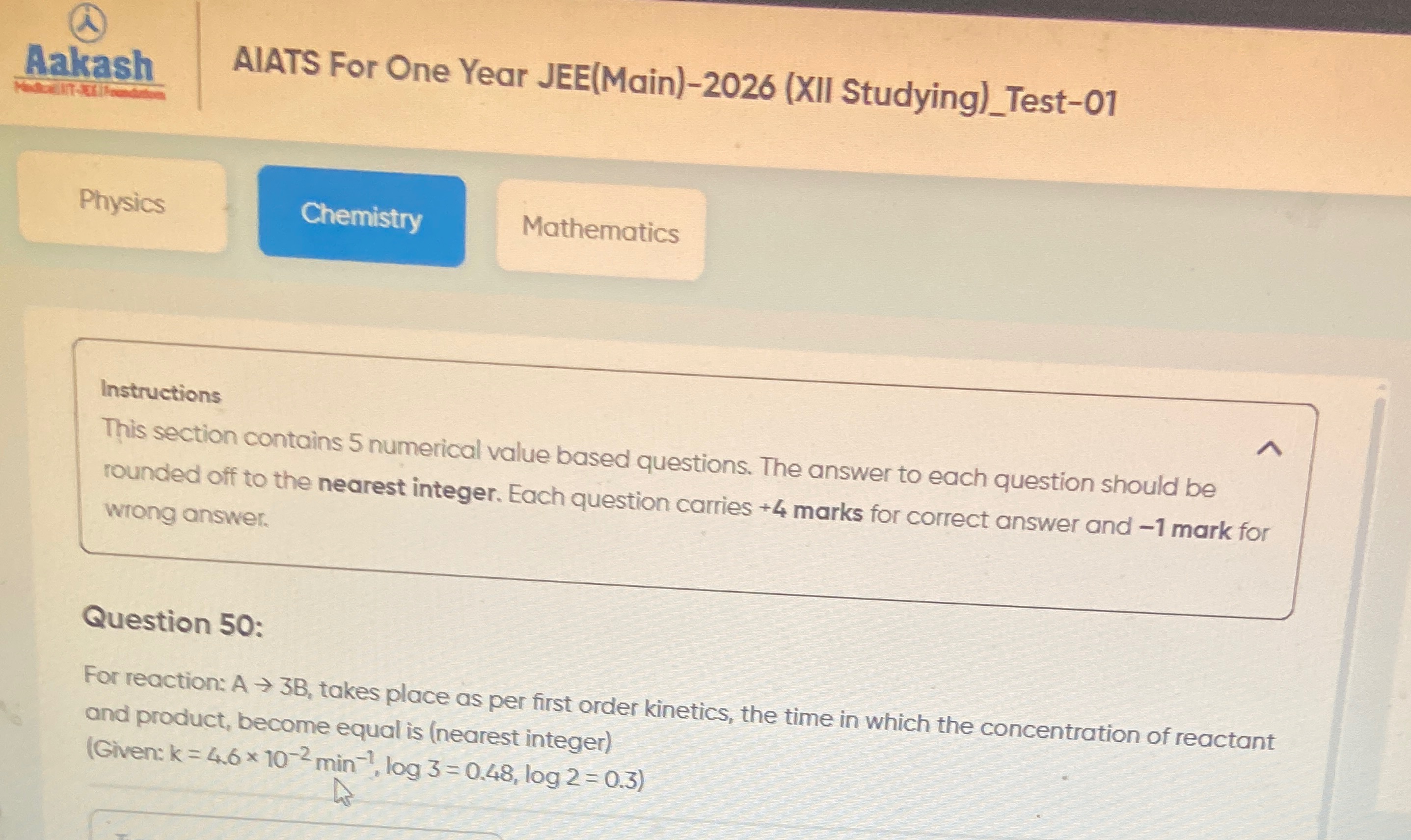
6
Solution
The problem asks for the time when the concentration of the reactant (A) and the product (B) become equal for a first-order reaction A → 3B.
1. Define initial and final concentrations:
Let the initial concentration of reactant A be [A]0.
At time t, let x be the concentration of A that has reacted.
According to the stoichiometry of the reaction A → 3B:
If x moles of A react, then 3x moles of B are formed.
The concentration of reactant A at time t will be:
[A]t=[A]0−x
The concentration of product B at time t will be:
[B]t=3x
2. Set up the condition for equal concentrations:
We are given that at time t, the concentration of reactant and product become equal:
[A]t=[B]t
Substitute the expressions for [A]t and [B]t:
[A]0−x=3x
[A]0=4x
From this, we can express x in terms of [A]0:
x=4[A]0
Now, find the concentration of A remaining at time t, [A]t, in terms of [A]0:
[A]t=[A]0−x=[A]0−4[A]0=43[A]0
3. Apply the integrated rate law for a first-order reaction:
For a first-order reaction, the integrated rate law is:
t=k1ln([A]t[A]0)
Substitute the expression for [A]t into the equation:
t=k1ln(3[A]0/4[A]0)
t=k1ln(34)
4. Substitute the given values and calculate t:
Given:
Rate constant, k=4.6×10−2 min−1
log3=0.48
log2=0.3
We need to convert the natural logarithm (ln) to base-10 logarithm (log):
lnx=2.303logx
So, the equation for t becomes:
t=k2.303log(34)
t=k2.303(log4−log3)
Since log4=log(22)=2log2:
t=k2.303(2log2−log3)
Now, substitute the numerical values:
t=4.6×10−22.303(2×0.3−0.48)
t=0.0462.303(0.6−0.48)
t=0.0462.303(0.12)
t=50.065217×0.12
t=6.007826 min
5. Round to the nearest integer: Rounding 6.007826 to the nearest integer gives 6.
The final answer is 6.
