Question
Question: \(AgN{O_3}\,\left( {aq} \right)\) was added to an aqueous \(KCl\) solution gradually and the conduct...
AgNO3(aq) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance (Λ) versus the volume of AgNO3 is
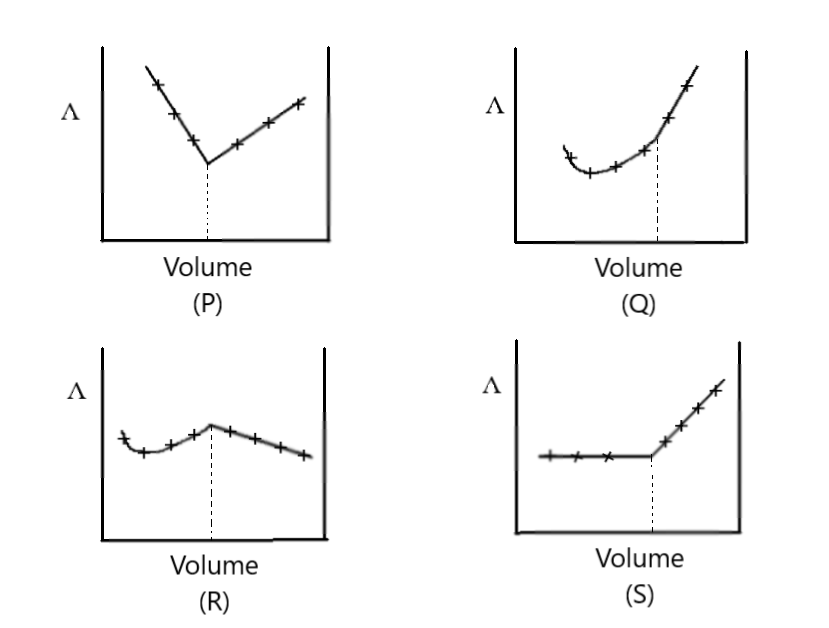
A) P
B) Q
C) R
D) S
Solution
The conductivity of a solution depends upon the quantity of ions at the same time on the mobility of ions present in the solution
Usually Cl− and NO3− have the same mobility hence both will have the similar effect on the value of conductance
Complete step by step solution:
The reaction for this question will be
AgNO3+KCl→KNO3+AgCl↓
Initially the solution consist only of two type of ions from KCl that are K+ and Cl−
Now when AgNO3 is added to this solution there will be four types of ions in the solution as follow
K+,Cl−,Ag+andNO3−
As we know that Ag+ will react with Cl− to form a white precipitate of AgCl
Now this will reduce the amount of Cl− but this decrease ions will be compensated by the NO3− ions which have the same mobility as Cl−
Now whatever the change happened in conductance value due to elimination of Cl− ions was compensated by NO3− ions hence the value of conductance remains constant for some time as respect to volume of AgNO3
After some time all the Cl− ions will be consumed by Ag+ for forming white precipitate of AgCl
And further addition of AgNO3will increase amount of Ag+ causing a rapid increase in the conductance value of solution
The reaction for this question will be
AgNO3+KCl→KNO3+AgCl↓
Hence, we find out that the conductivity of the solution initially remain constant but after excess addition of AgNO3it increased rapidly
Thus, the option is “D” the correct solution for the given question.
Note:
Further the conductivity of a solution also depends upon the size of the ion, on the dielectric constant and viscosity of solvent and the temperature of the solution
The S.I unit for measurement of conductance is Siemens (S)
