Question
Question: After np-orbitals are filled, the next orbital filled will be: (A) (n+1) s (B) (n+2) p (C) (n+...
After np-orbitals are filled, the next orbital filled will be:
(A) (n+1) s
(B) (n+2) p
(C) (n+1) d
(D) (n+2) s
Solution
The filling of electrons into the orbitals of different atoms takes place according to the aufbau principle. Aufbau principle states that in the ground state of an ion, electrons fill atomic orbitals of lowest available energy levels before occupying higher levels.
Complete answer:
The word aufbau in German means ‘building up’. It means filling up orbitals with electrons.
Orbitals of atoms are filled in order of increasing energies at ground state. Order of energies of orbitals are observed as.
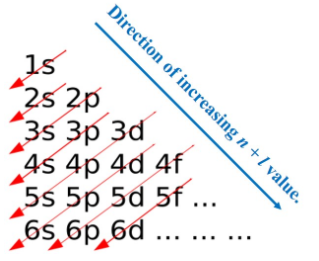
In the above figure we can see that electrons enter the s-orbital of the next energy level after p-orbital.
Electron will enter in (n+1) s orbital after p orbital.
Therefore, from the above explanation the correct option is (A) (n+1) s.
Additional Information: Let us explain Aufbau principle of filing of electrons by giving an example of copper.
Atomic number of copper is 26.
Electronic configuration is Cu is, 1s22s22p63s23p64s23d6.
As we shown in above figure energy of atomic orbital is in order of
1s < 2s < 2p < 3s < 3p < 4s < 3d
Since energy of atomic orbital 4s is less than 3d orbital therefore electron enters in 4s orbital.
Capacity of s-orbital is 2, therefore after filling s-orbital, remaining 6 electrons enter in next orbitals.
Note: Ideally, 4s should be filled after 3d. But we can observe that, in real, 4s is filled before 3d because the 3d orbital is having higher energy than 4s.
According to Hund’s Rule of maximum multiplicity.
If two or more orbitals of equal energy are available electrons will occupy them single before filling them in pairs.
