Question
Question: After completion of the reaction (I and II), the organic compound (s) in the reaction mixture is (ar...
After completion of the reaction (I and II), the organic compound (s) in the reaction mixture is (are):
Reaction 1:
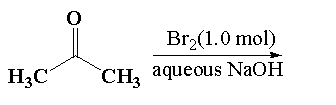
Reaction 2:
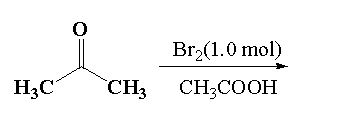
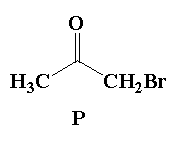
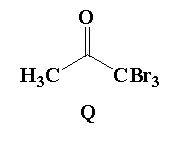
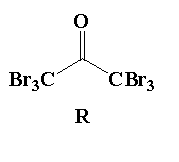
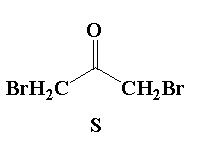
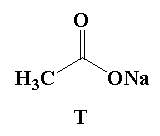
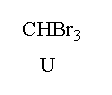
A. Reaction I: P and Reaction II: P
B. Reaction I: U, acetone and Reaction II: Q, acetone
C. Reaction I: T, U, acetone and reaction II:P
D. Reaction I: R, acetone and reaction II: S, acetone
Solution
In the given reaction, two reactions are taking place first the acetone is reacting with bromine in the basic medium and in the second reaction the acetone is reacting with bromine in the acidic medium. In basic medium haloform reaction is taking place and in the acidic medium replacement of alpha hydrogen is taking place.
Complete step by step answer:
It is given that in reaction 1, acetone reacts with 1.0 mol of bromine in presence of an alkaline medium using an alkaline solution of sodium hydroxide. This reaction is called a haloform reaction. In haloform reaction. Methyl ketone is reacted with bromine, chlorine or iodine in presence of an alkaline medium containing hydroxide ions OH−to give carboxylate ions and haloform reactions.
Here, in this question acetone reacts with 1.0 mole of bromine in presence of sodium hydroxide to give sodium salt of acetic acid and a bromoform.
The reaction is shown below.
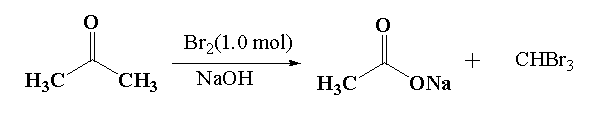
In the given mixture T is the sodium acetate and U is the bromoform.
It is given that in reaction 2, acetone reacts with 1.0 mol of bromine in presence of acetone (acidic medium). In this reaction the alpha hydrogen of acetone is replaced by the bromine atom to form alpha bromoacetone.
The reaction is shown below.
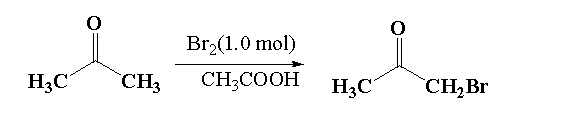
In the given mixture, alpha bromo acetone is P.
Therefore, the correct option is C.
Note:
Aldehyde does not give any haloform reaction but there is an exception in aldehydes. The only aldehyde which gives haloform reaction is acetaldehyde.
