Question
Question: Addition of excess potassium iodide solution to a solution of mercuric chloride gives the halide com...
Addition of excess potassium iodide solution to a solution of mercuric chloride gives the halide complex:
A.TetrahedralK2[HgI4]
B.TrigonalK2[HgI3]
C.LinearHg2I2
D.Square planarK2[HgCl2I2]
Solution
Hint:
To solve this question, first write the equation for the reaction. The given equation is an example of double displacement reaction. Then, through electronic configuration of the central atom of the complex, decipher the geometry.
Complete step by step answer:
Let us write the equation for the reaction given in the question.
According to the question, mercuric chloride reacts with excess potassium iodide solution to give a halide complex. Therefore,
HgCl2+4KI→K2[HgI4]+2KCl
So, the halide complex formed is Potassium mercuric iodide - K2[HgI4]. This is an example of double displacement reaction.
Now, let us use the geometry of the complex.
Mercury is the central compound of the complex. The electronic configuration of Hg is –
Hg=[Xe]4f145d106s2
In the complex, mercury has an oxidation state of +2.
Hg2+=[Xe]4f145d106s0
It therefore has orbitals 6s and 6p for bonding.
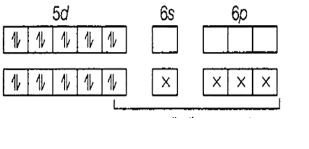
Since bonding takes place through 1 s-orbital and 3-p orbitals, the hybridization of the compound is sp3. All sp3hybridized compounds have a tetrahedral geometry.
Therefore, the answer is – option (a).
Additional Information: The IUPAC name of the halide formed is Potassium tetraiodomercurate(II).
Note:
The hybridization and geometry of any compound can be related as –
| Hybridization | Geometry |
|---|---|
| sp | Linear |
| sp2 | Trigonal planar |
| sp3 | Tetrahedral |
| sp3d | Trigonal bipyramidal |
| sp3d2 | Octahedral |
| sp3d3 | Pentagonal bipyramidal |
