Question
Question: Action of soap is due to emulsification of micelle formation. Comment....
Action of soap is due to emulsification of micelle formation. Comment.
Solution
Soap is sodium or potassium salt of fatty acids. It is produced by hydrolysis of fats in a chemical reaction called saponification. It consists of two parts, tail and head. Tail is made of a long hydrocarbon chain and the head is a carboxylate part.
Complete step by step answer:
Soap is basically sodium and potassium salt of long chain fatty acid RCOO−Na+ . End of the molecule to which sodium ion is attached is polar in nature that means it will dissolve in water as water is also polar in nature. As it dissolved in water it is called the hydrophilic part means water loving. Alkyl chain is non polar hence it does not dissolve in water. Due to this reason it is called the hydrophobic part which means water repulsive.. When soap is added to water containing dirt then the hydrophobic part (non-polar) gets attached to dirt and the hydrophilic part (polar part) remains on the water side that is away from dirt. This forms a circular arrangement around a dirt molecule. This is called micelle formation. This means the polar part dissolves in water and the non polar part dissolves in dirt. Micelles are negatively charged so they will not coalesce and a stable emulsion will be formed.
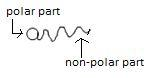
This picture shows the polar and nonpolar part of the soap molecule.
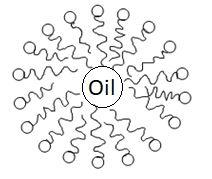
In this picture we can see that the nonpolar part that is hydrophobic is attached to a dirt molecule and the polar part that is hydrophilic is outside the water. Hydrophilic part tends to remain in water and the hydrophobic part tends to stick with dirt molecules. This results in micelle formation.
This picture shows micelle formation.
Additional information: Emulsions are used in various fields such as:
Cleansing action of soap is based on formation of emulsions.
Digestion of fats inside our intestine takes place by process of emulsification.
This process is used in making medicines.
Antiseptics and disinfectants also form emulsions when dissolved in water.
Note:
In case of soap remember two words hydrophilic and hydrophobic. Hydrophilic means water loving and hydrophobic means water hating. These two parts are responsible for the cleansing mechanism of soap, micelle formation and emulsification.
