Question
Question: Acidified potassium permanganate is decolourised by A.Bleaching powder B.White vitriol C.Mohr’...
Acidified potassium permanganate is decolourised by
A.Bleaching powder
B.White vitriol
C.Mohr’s salt
D.Microcosmic salt
E.Laughing gas
Solution
We need to know that the potassium permanganate chemical formula is KMnO4 , it is an inorganic compound. In the chemical industry and laboratories potassium permanganate is widely used. In the laboratories KMnO4 is used as a strong oxidizing agent in many reactions, because potassium permanganate contains MnO4− ions.
Complete step by step answer:
As we know that, potassium permanganate is composed of K+ and MnO4− . Potassium permanganate is used in many titrations as an oxidizing agent.
We can draw the potassium permanganate structure as,
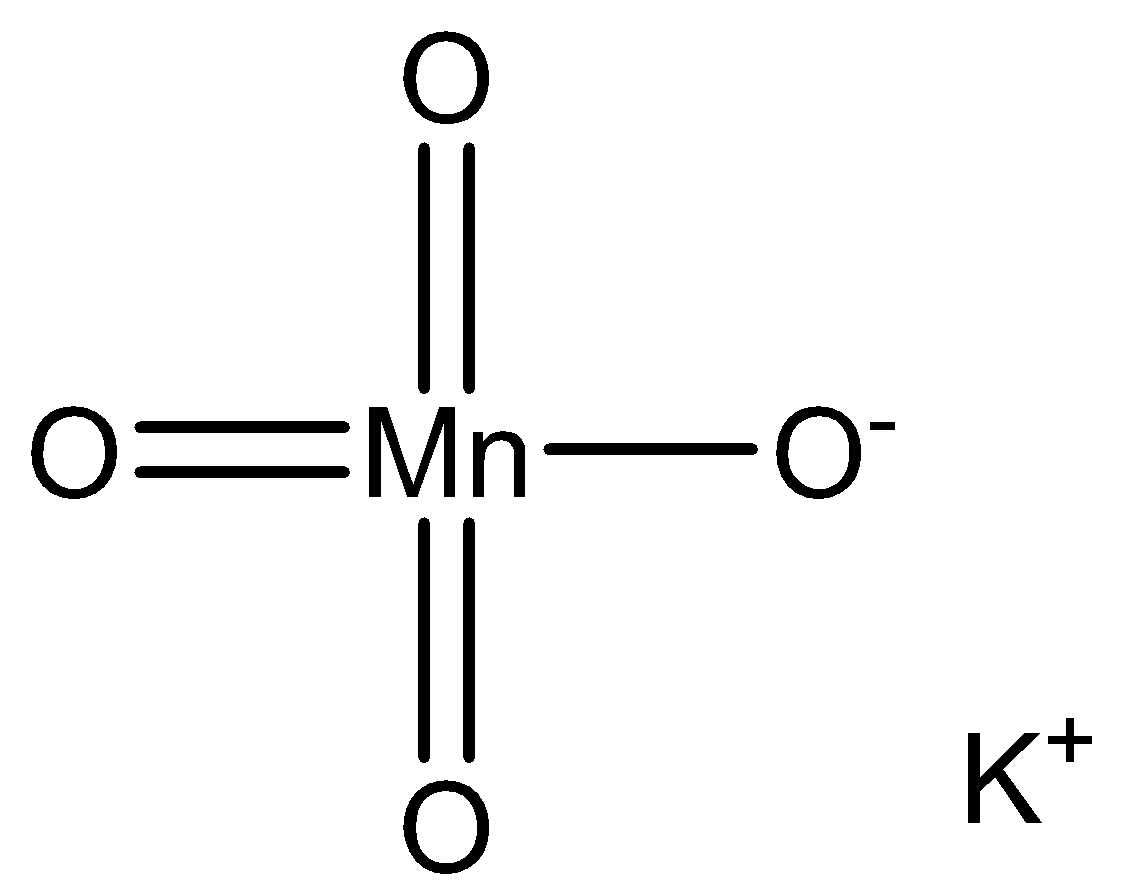
We use acidified potassium permanganate. The aicd is sulphuric acid. The acid prevents hydrolysis. Acid provides protons and it is necessary to carry a reaction. The equation for the reaction is,
2KMnO4+3H2SO4→K2SO4+2MnSO4+3H2O+5[O]
Acidified potassium permanganate decolourised equation is,
(2FeSO4(NH4)2SO4.6H2O+H2SO4+5[O]→Fe2(SO4)3+2(NH4)2SO4+13H2O)×5
The overall equation for above two equation is,
2KMn{O_4} + 10FeS{O_4}{(N{H_4})_2}S{O_4}.6{H_2}O \to K_2SO_4 + 2MnS{O_4} + 5F{e_2}{(S{O_4})_3} + 10{(N{H_4})_2}S{O_4} + 68{H_2}O$$$${(N{H_4})_2}Fe{(S{O_4})_2}{({H_2}O)_6} is known as ammonium iron (II) sulphate and it is used for decolourise acidified potassium permanganate.
We must need to know that the ammonium iron (II) sulphate is also known as Mohr’s salt. Mohr was a German scientist.
From the above data Mohr’s salt is used to decolourise acidified potassium permanganate.
So C. Mohr’s salt is the correct answer.
Additional information:
We must need to remember that for detecting halides, oxalates, sulphates, etc. detected in qualitative analysis with the use of potassium permanganate. Potassium permanganate is also used in medicines and also used as disinfectant. Baeyer reagent is the alkaline solution of potassium permanganate.
Note:
We need to know that in the compounds of Mn(VII) , potassium permanganate (KMnO4) is the most important compound. Potassium permanganate color is purple due to the permanganate ion, MnO4−. Potassium permanganate is reduced to manganese (II) ion in acidic solution. The equation for the reaction is,
2KMnO4+3H2SO4→K2SO4+2MnSO4+3H2O+5[O]
For an organic compound acidified potassium permanganate is also used as an oxidizing agent.
