Question
Question: Acetylene is acidic and hence it reacts with \(NaOH\) or \(KOH\)....
Acetylene is acidic and hence it reacts with NaOH or KOH.
Solution
Here answer to this question is to be stated as whether true or false and for this the answer lies in fat fact about whether acetylene is acid or not which is the base given in the chapters of chemistry.
Complete step by step answer:
In the lower classes of chemistry, we have come across the concept that tells us about acidity and basicity and their variation given by the dissociation constants.
Now, let us see whether acetylene can react with the alkali compounds that are NaOH and KOH.
To start with, acetylene is given by the structure and this acid when reacts with a strong base it forms a conjugate base by donating a proton to base because base abstracts a proton. That is as shown below,
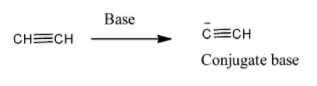
The pKa value for acetylene is 25 and this shows that it is a very weak acid and therefore it does not give conjugate base.
From this, we can understand that with the big bases there occurs no reaction and also with NaOH or KOH because they are also weak bases. But, acetylene reacts with NaNH2 which is a very strong base and produces a conjugate base.
Therefore, the correct answer is that the given statement is false.
Note: Not all acids can react completely with base and this depends on the types of base present. The weak acid reacts with strong bases as it can form a conjugate base and weak base reacts with strong acids to form conjugate acids. Thus, this point is important while solving this type of question.
