Question
Question: Acetyl bromide reacts with an excess of CH3MgI followed by treatment with a saturated solution of N...
Acetyl bromide reacts with an excess of CH3MgI followed by treatment with a saturated solution of NH4Cl gives:
A. acetone
B. acetamide
C. 2 - methyl - 2 – propanol
D. acetyl iodide
Solution
To answer this question, you should recall the concept of preparation of alcohols. The simplest method to produce all primary, secondary and tertiary alcohol is by using a Grignard reagent.
Complete step-by-step answer: We know that to produce primary alcohol, the Grignard reagent is reacted with formaldehyde.

Methyl Magnesium Formaldehyde Ethanol
Bromide
Further reacting a Grignard reagent with any other aldehyde will lead to a secondary alcohol.
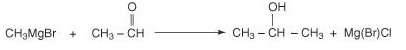
Acetaldehyde 2-propanal
Ultimately, reacting a Grignard reagent with a ketone will generate tertiary alcohol.
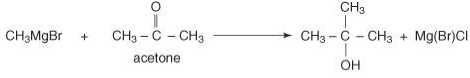
tert-butyl alcohol
For the reaction given in the question: Acetyl bromide reacts with an excess of CH3MgI followed by treatment with a saturated solution of NH4Clgives tertiary alcohol which in the given option is 2-methyl-2-propanol. The mechanism can be visualized as follows:
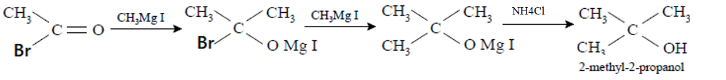
Therefore, we can conclude that the correct answer to this question is option C.
Note: Grignard reagent refers to an organo-magnesium halide with the formula of RMgX, where X is a halogen, and R is an alkyl or aryl. Apart from preparation of alcohols, it is also used for determining the number of halogen atoms present in a halogen compound. Currently, another important use involves the chemical analysis of certain triacylglycerols.
