Question
Question: Acetone reacts with iodine \(({I_2})\) to from iodoform, in the presence of : (A) \[KOH\] (B) \[...
Acetone reacts with iodine (I2) to from iodoform, in the presence of :
(A) KOH
(B) NaOH
(C) CuCO3
(D) MgCO3
Solution
Iodoform is chemically tri-iodo-methane its chemical formula is CHI3.
Iodoform is prepared by ethyl alcohol and acetone in laboratories.
Complete step by step answer:
(1) Acetone is when treated with iodine and potassium hydroxide, producing iodoform.
When reaction takes place in following steps.
(2) In the first step iodine reacts with acetone in presence of KOH and form tri-iodo-acetone.
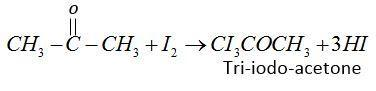
(3) Second step tri-iodo-ethane reacts with KOHand forms iodoform.
CI3COCH3+KOH→CHI3+CH3COOK
Iodoform potassium acetate
Therefore, form the above explanation the correct option is (A) KOH.
About 3.5mlof acetone is taken in a flask. Powdered iodine is added to the flask, stir the mixture and place this mixture in a warm water bath.
Temperature is maintained at 70∘cto 80∘c.
When a solution is cooled yellow crystals of iodoform are obtained.
This method is an identification test for the presence of ketone groups in organic compounds.
This is also known as iodoform test.
Only methylated ketone shows this reaction.
If it means one of the alkyl groups in ketone should be methyl group.
So, the correct answer is “Option A”.
Additional Information:
Iodoform can also be prepared by ethanol. When ethyl alcohol is heated with iodine and sodium hydroxide or aqueous Na2CO3 form yellow crystalline solid iodoform.
CH3CH2OH+CI2+6NaOHΔCHI3+HCOONa+5NaI+5H2O
Iodoform is a crystalline, volatile substance.
It has a distinctive odor and halogens to chloroform.
This is used as an antiseptic dressing.
Note:
As we discussed above only methylated ketones give iodoform test.
3-pentanone
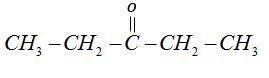
Does not give iodoform test because it has two ethyl groups attached to carbonyl groups.
An aldehyde or ketone which has a methyl group attached to a carbonyl group will give a positive test.
