Question
Question: Acetone(\(CH_3COOH\)) is the major product in: I.  is the major product in:
I. 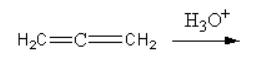
II. 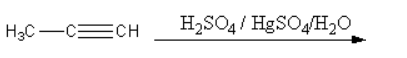
III. 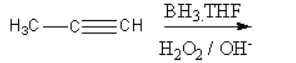
A. I
B. II.
C. III.
D. None of the above
Solution
The alkene and alkyne work as nucleophiles and attacks on acid forming a cation. The reagent H2SO4/HgSO4 cause mercuration and demercuration. The reagent BH3.THF is an electrophile. This reagent breaks the triple bond of alkyne and gets attached. The hydrolysis of this attaches to the hydroxyl group at terminal carbon.
Complete step by step answer:
The mechanism of I-reaction is as follows:
First the reactant attacks on the hydronium ion, so carbocation forms. On this carbocation, oxygen atoms of water attack and get attached. Then by the removal of hydride enol forms. The enol can be converted into keto form by keto-enol tautomerism.
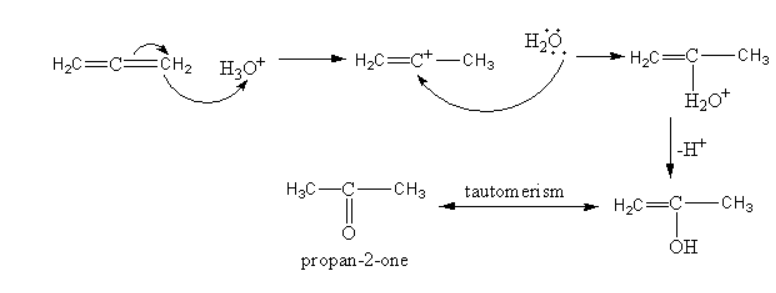
So, the product of reaction-I is a ketone that is propanone.
The mechanism of II-reaction is as follows:
First the mercury attacks on the reactant and forms a cyclic structure. Then water attacks on this cyclic structure forming a mercury cation. Then by the removal of hydride alcohol forms. The enol can be converted into keto form by keto-enol tautomerism. The oxygen atom of the ketone attacks on the acid and gets protonated and the diacation of mercury removes. The enol form again converts into keto form by keto-enol tautomerism.
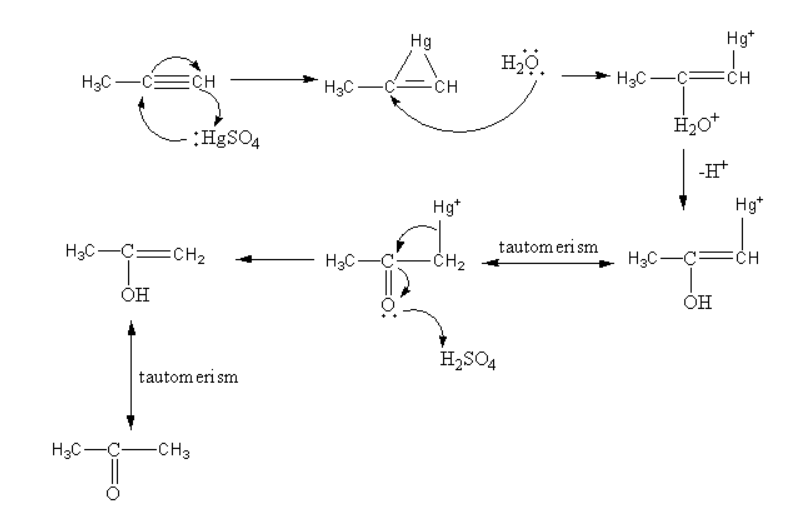
So, the product of reaction-I is a ketone that is propanone.
The mechanism of III-reaction is as follows:
The nucleophilic alkyne attacks on electrophilic boron atoms. Then the hydrolysis takes place forming an alcohol. The alcohol shows keto-enol tautomerism and forms keto form that is aldehyde.
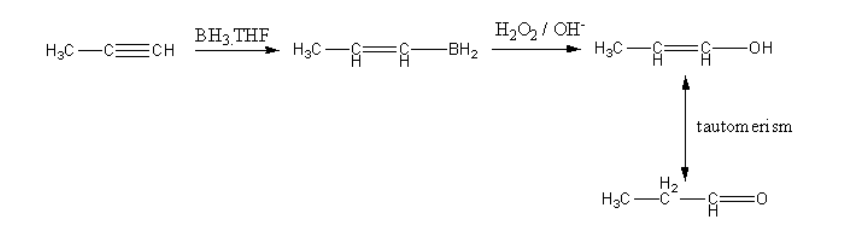
So, in reaction-I and reaction-II, acetone is formed as a product.
**Therefore, option (A) I and option (B) II, both are correct.
Note: **
In reaction-II mercury attaches with the reactant in between the reaction mechanism and then removes. The reaction-II is known as oxymercuration demercuration. In reaction-III, the given alkyne is terminal. The terminal alkyne gives aldehyde. The non-terminal alkyne gives ketone with this reagent. The reaction-III gives anti-markovnikov products.
